Seamless Transition from Stability Data to automated Submission-Ready Reports
Submission-ready reports in minutes: Automate stability studies with our purpose-built reporting solution for pharmaceutical quality processes and documentation. Transform time-consuming manual workflows into efficient, GxP-compliant reporting procedures.
Seamless Transition from Stability Data to automated Submission-Ready Reports
Submission-ready in minutes: Automate stability studies with our purpose-built reporting solution for pharmaceutical quality processes. Transform time-consuming manual workflows into efficient, GxP-compliant reporting procedures.
Comprehensive Stability Reporting Capabilities
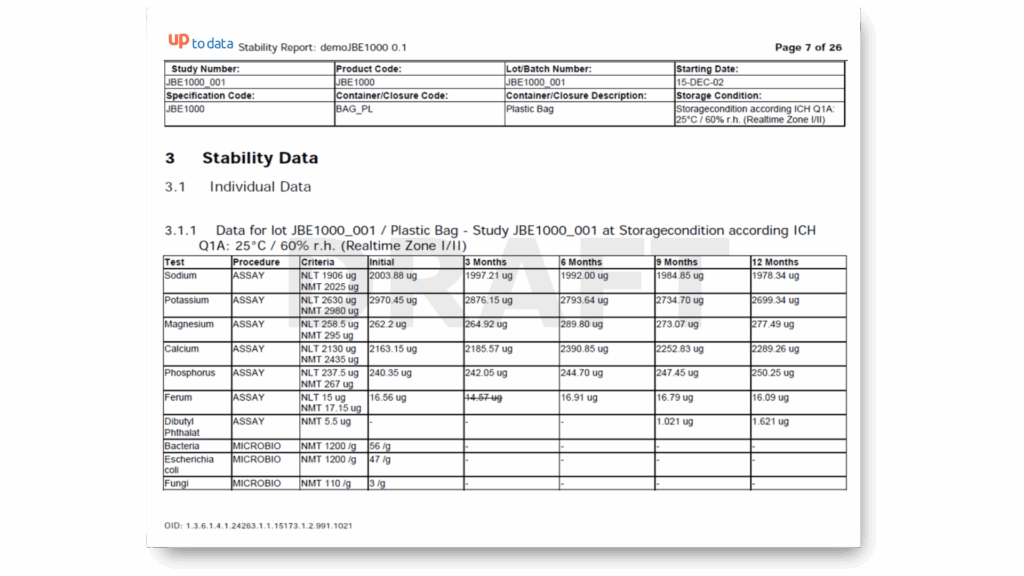
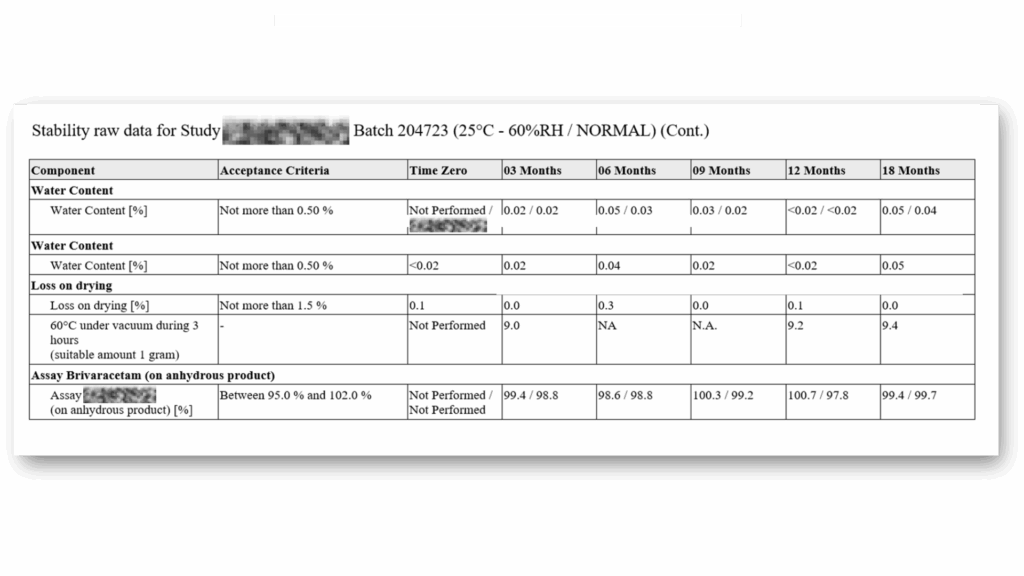
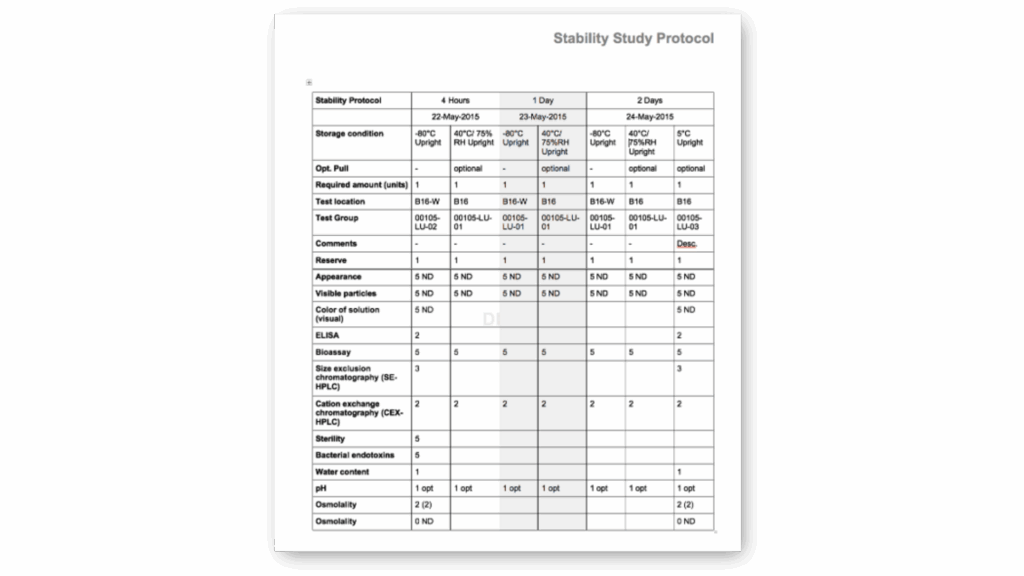
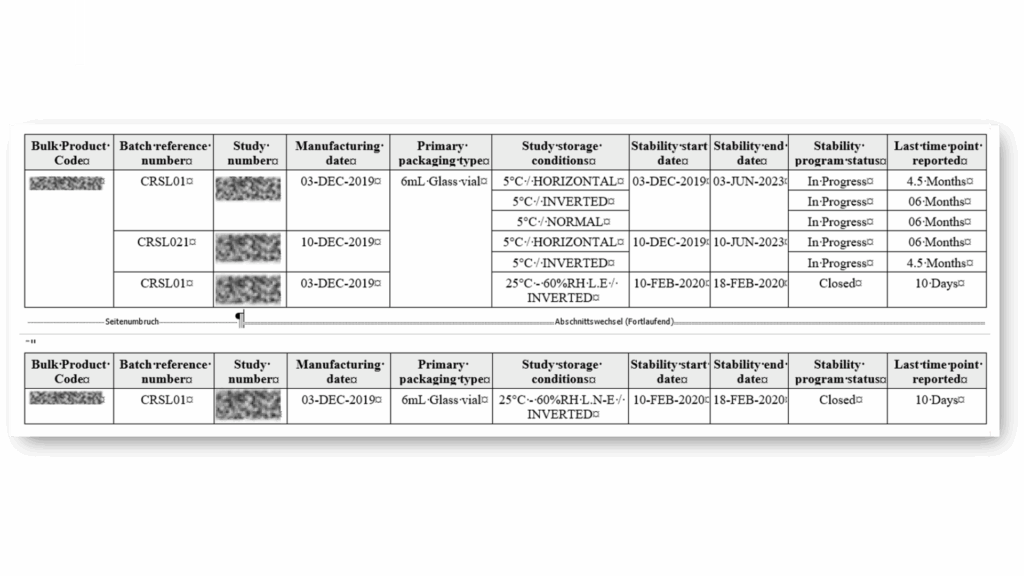
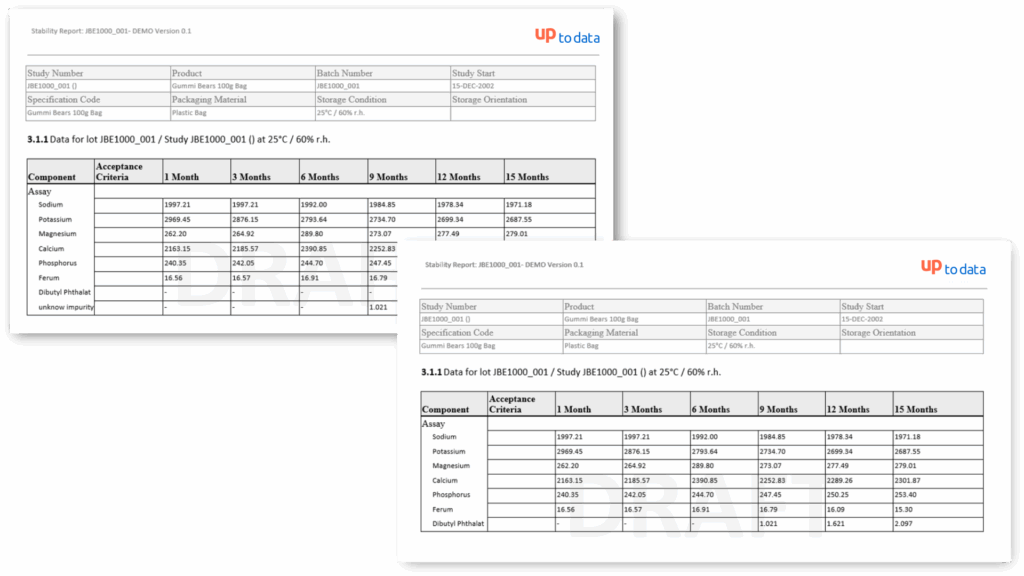
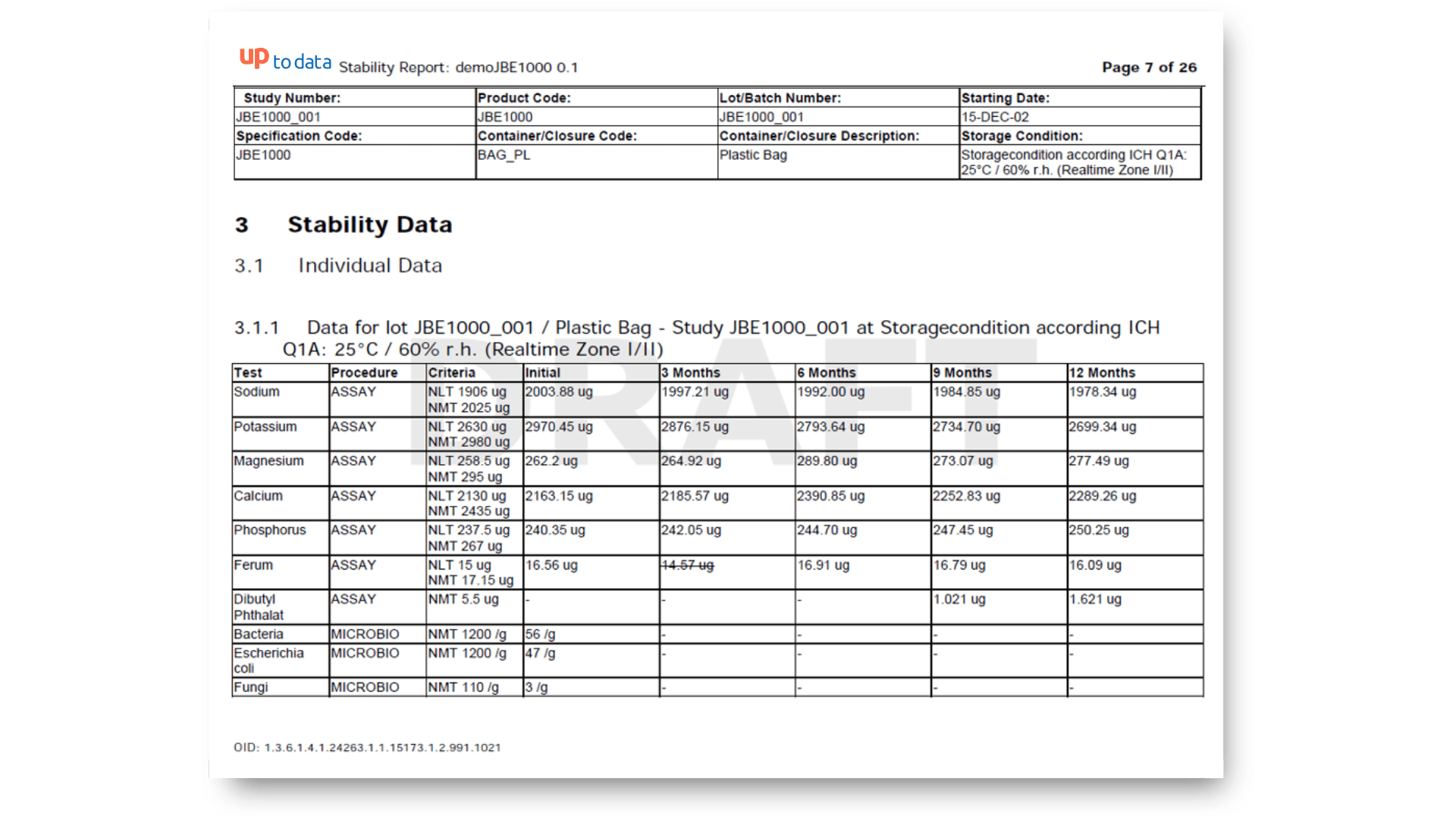
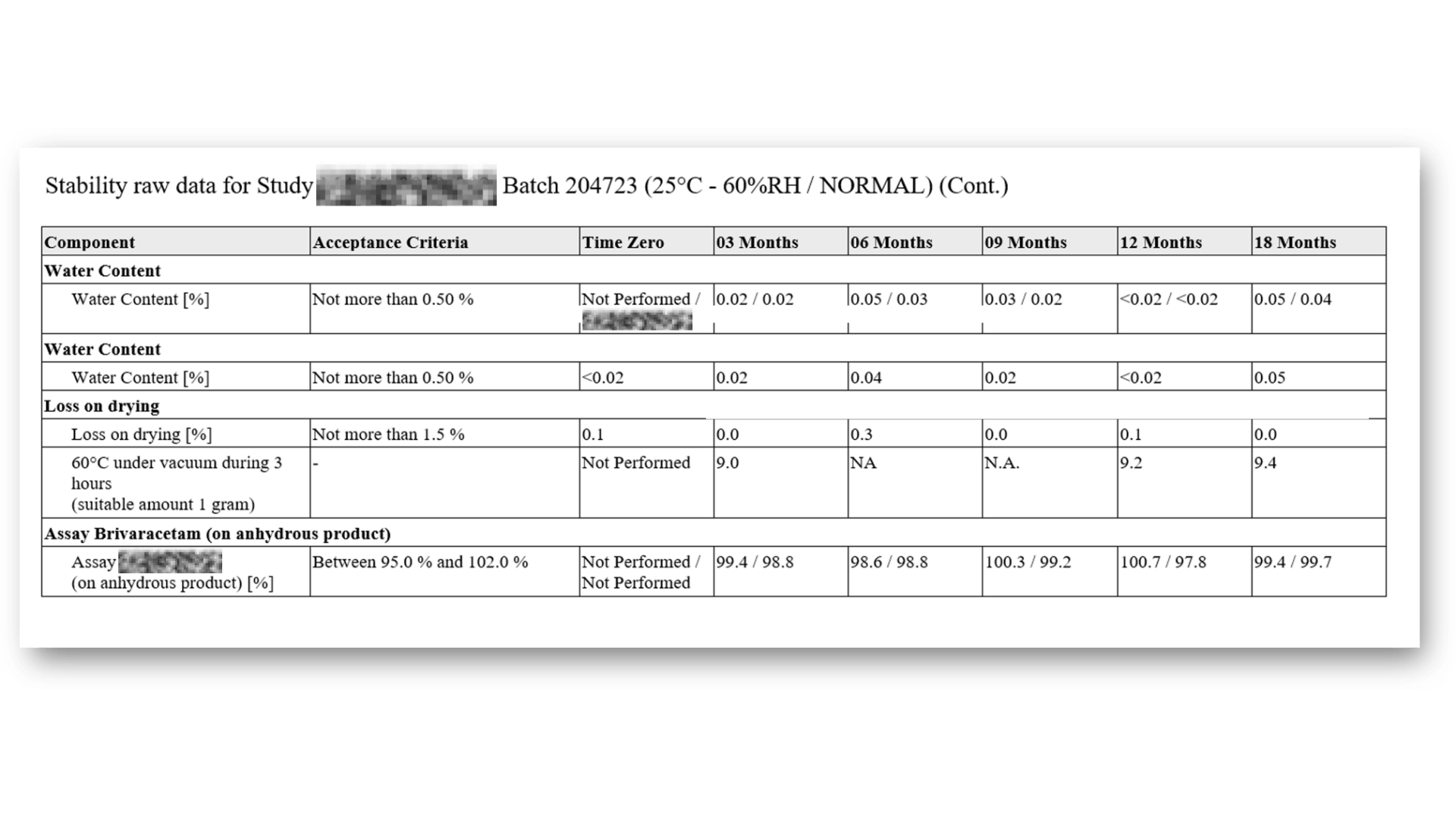
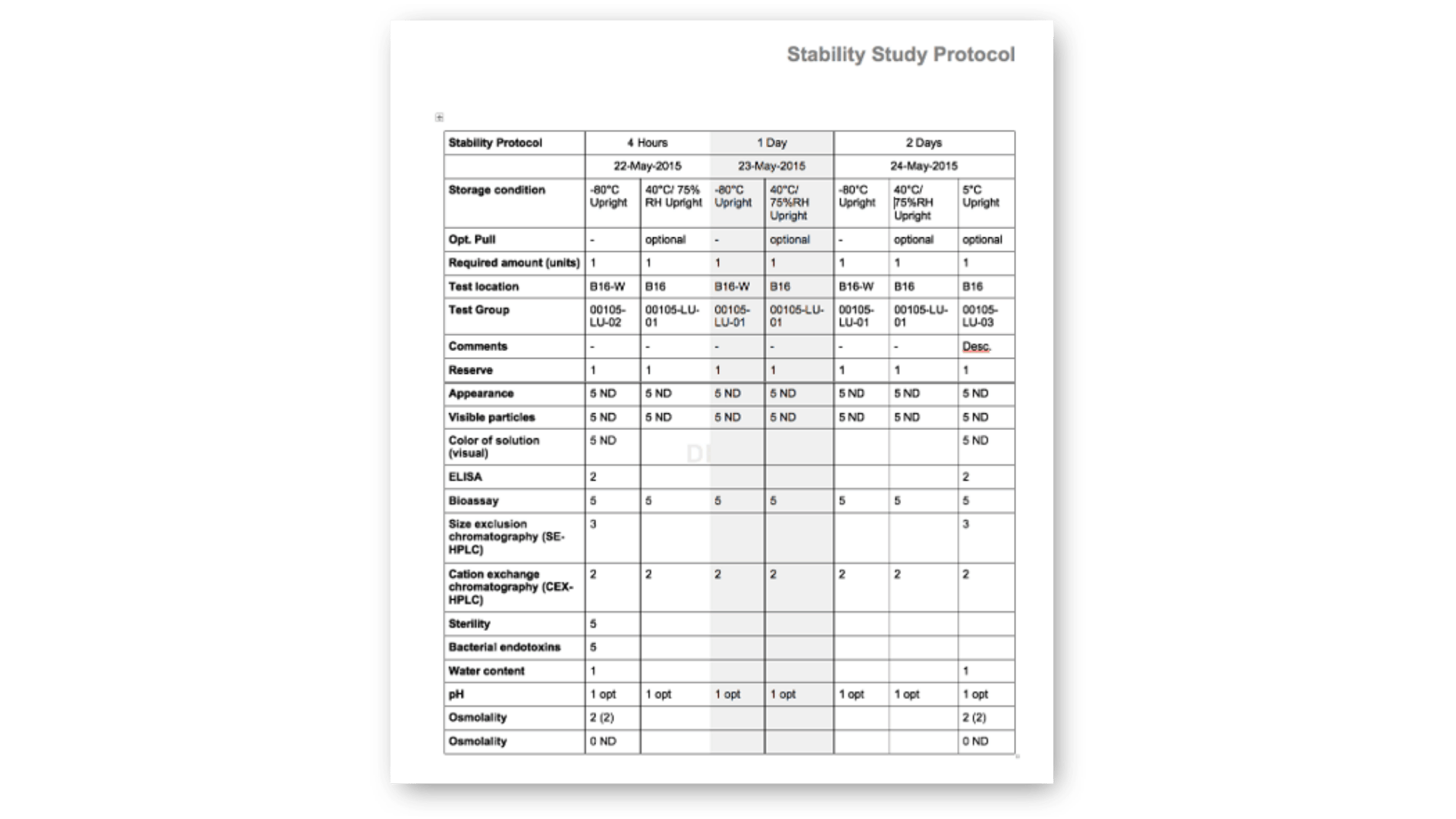
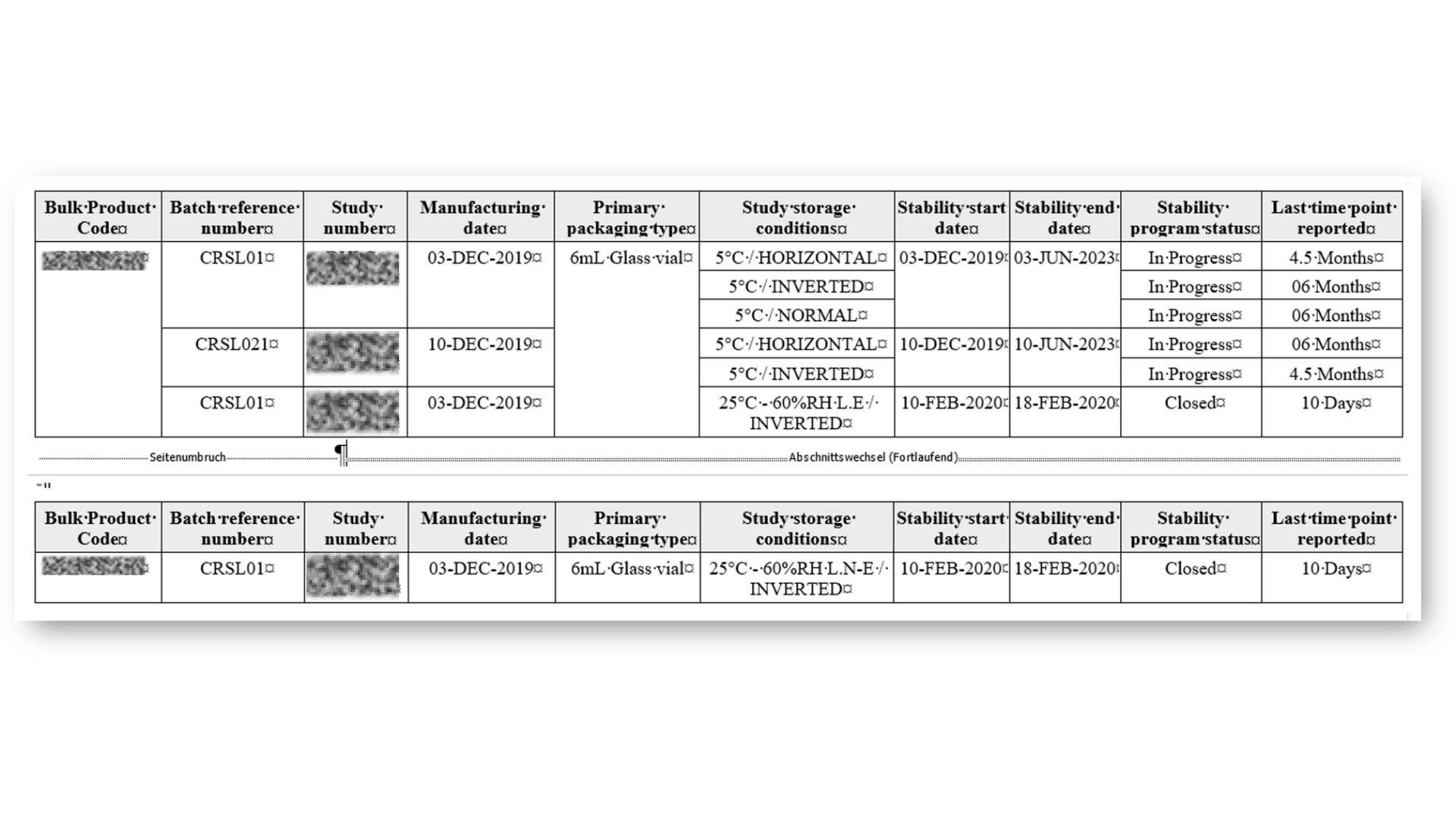
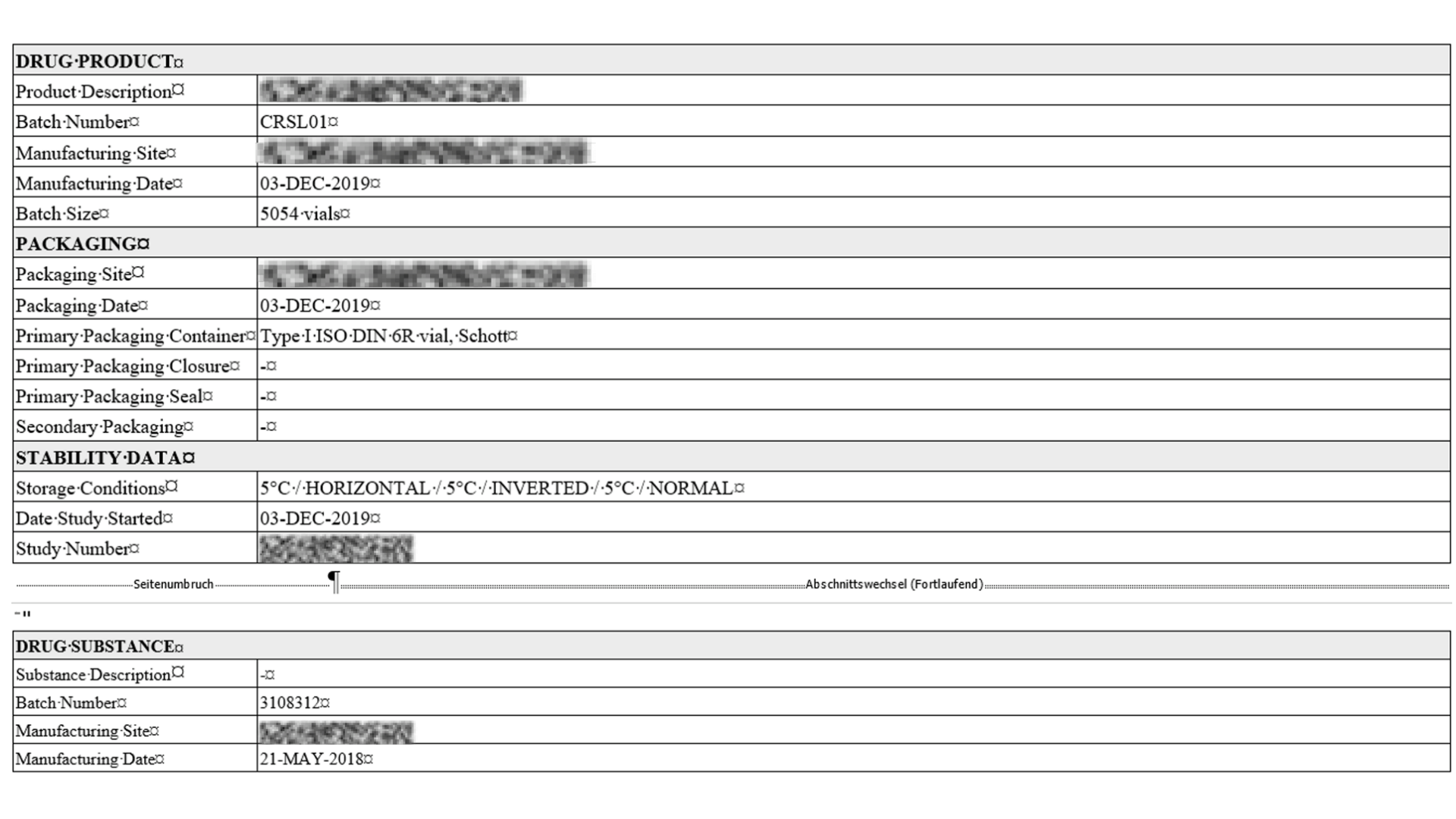
Standard Stability Tables included
Generate all kind of stability tables with automated data transformation from various LIMS sources, ensuring data integrity and regulatory compliance throughout the automated reporting process.
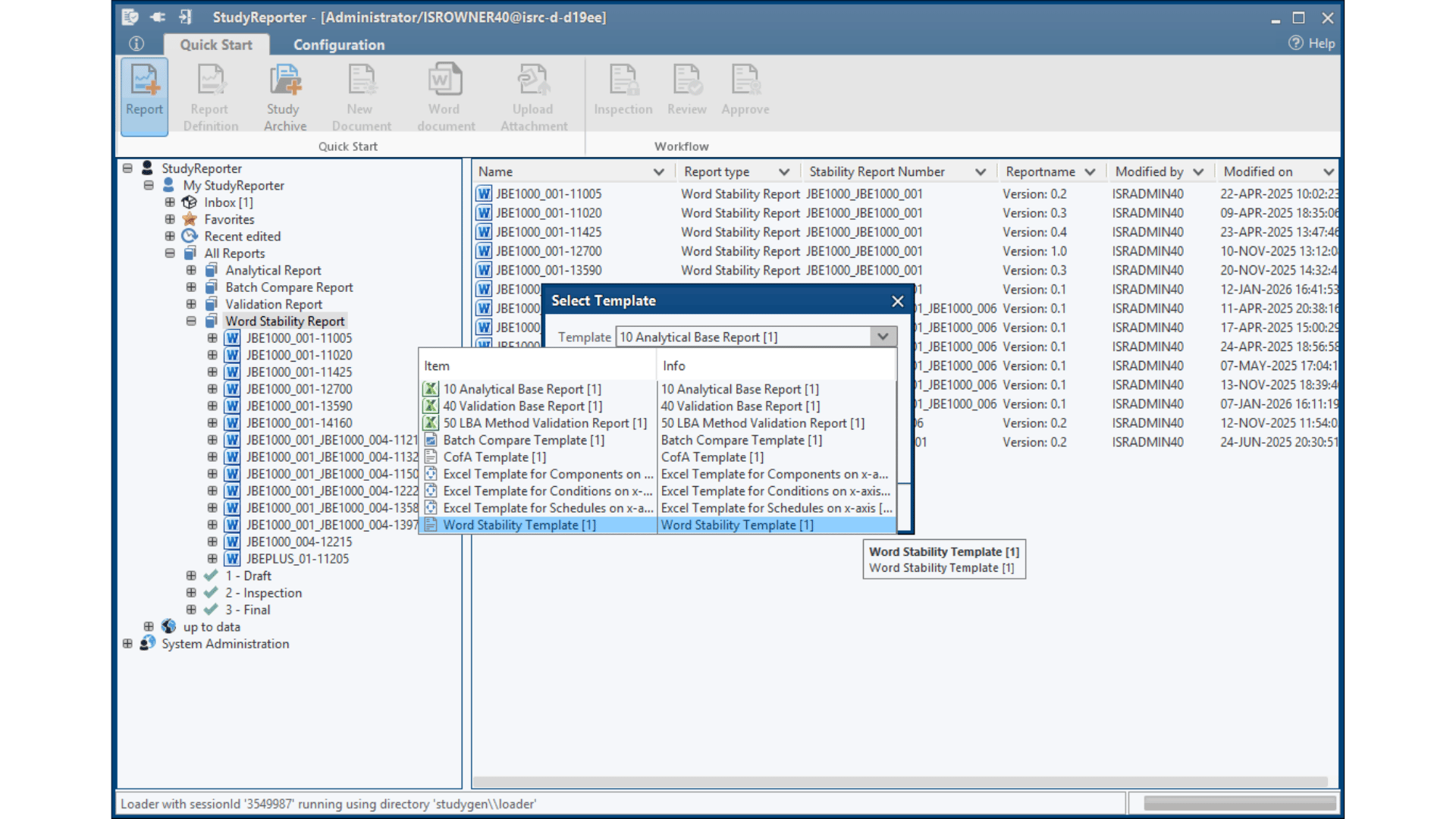
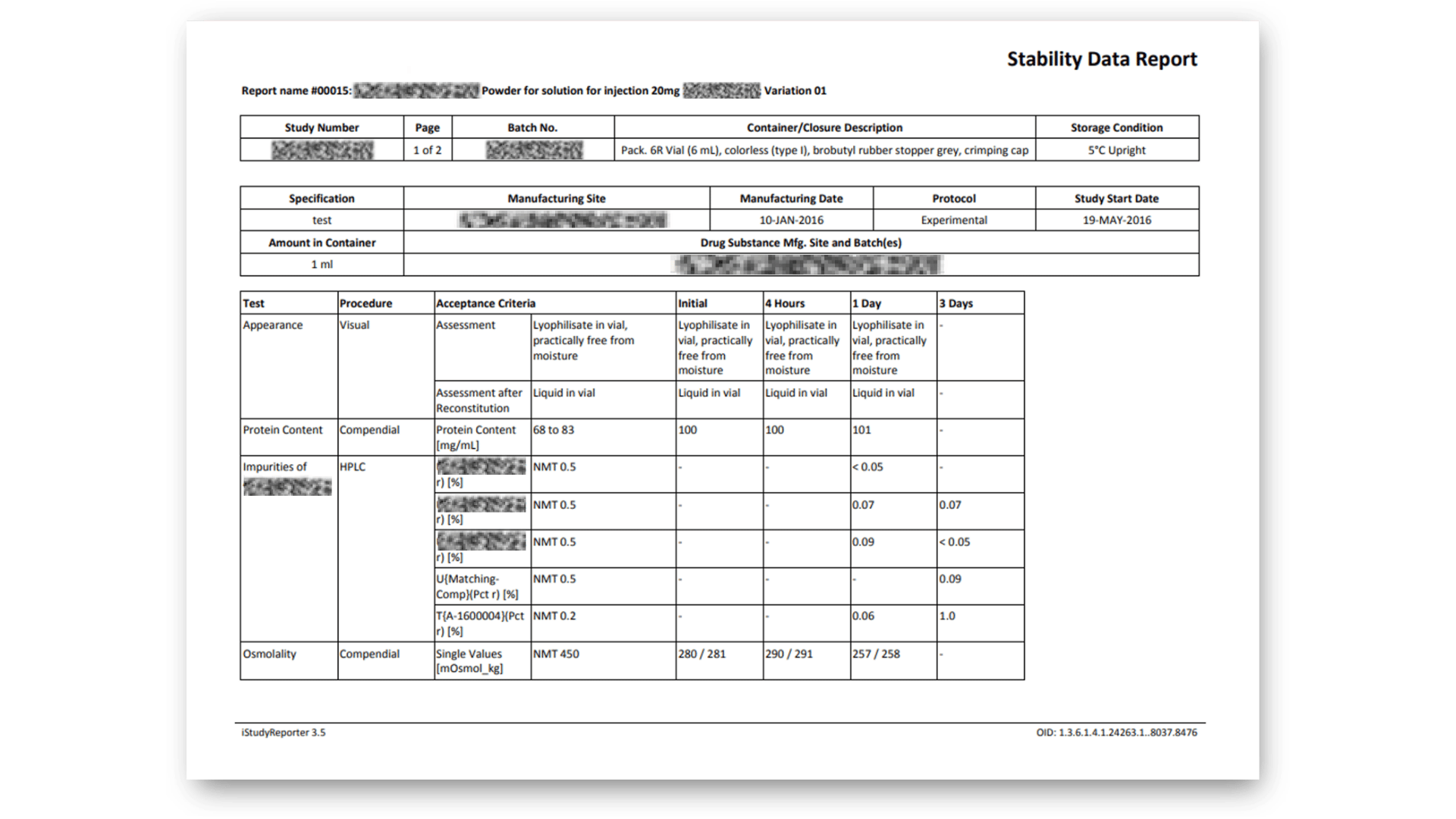
Configurable Report Templates
Configure your template for different purposes – from CofA and initial approval to ongoing stability studies, and ad-hoc reports. Perfect-fit for every stability study phase.
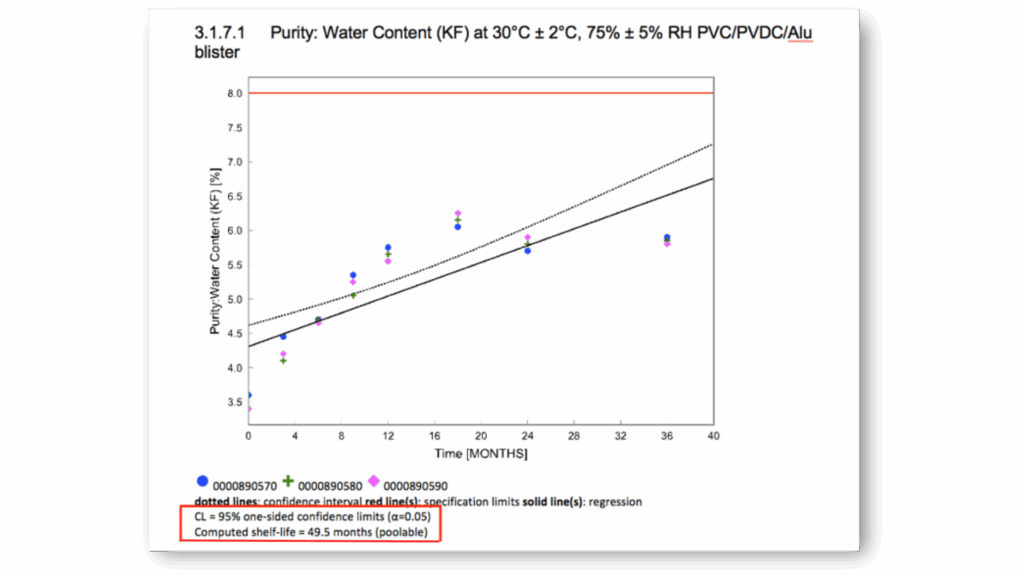
ICH Q1E Shelf-Life Evaluation
Automated shelf-life and shelf-life plots determination following ICH Q1E guidelines, with comprehensive statistical modeling and confidence interval calculations – immediately ready for your approval documentation without any further external systems.
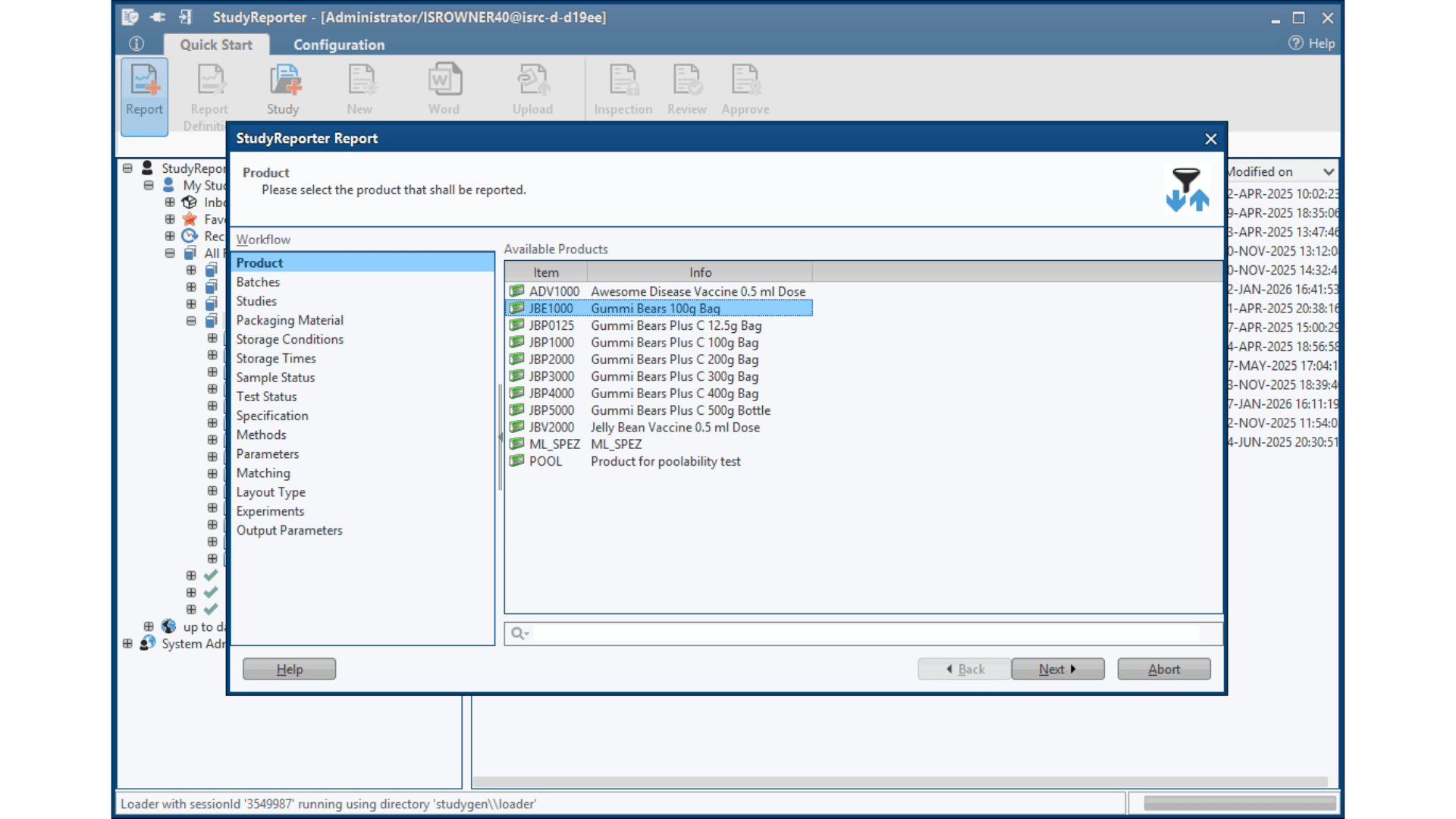
Seamless System Integration
Secure and validated interfaces to LIMS, ELNs, SAP, and other laboratory database systems, ensuring compliant data transfer across your entire digital ecosystem. Specific connectors and automated data mapping, eliminate manual data entry while maintaining full audit trails and 21 CFR Part 11 compliance throughout the reporting process.
Unknown Impurity Control Strategy
Unknown impurities, typos in parameter names, changed method definitions, or country-specific product specs? No problem – StudyReporter Stability catches and fixes all of that right during report creation.
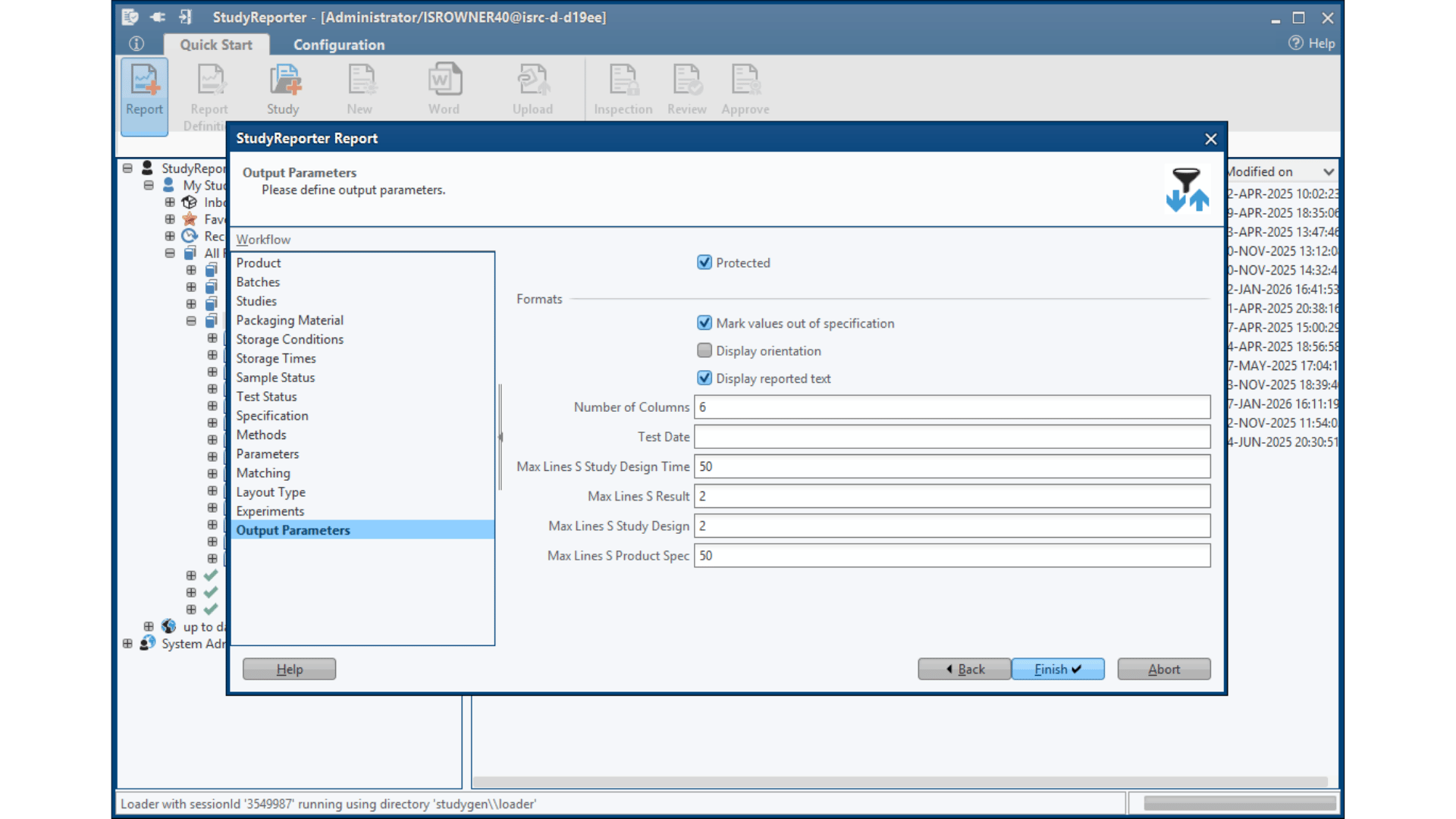
Submission-Ready Output
Equipped with pre-configured report templates for all major regulatory submissions, including INDA, NDA, Annual Reports, and PQR/APR. This ready-to-use framework ensures compliance with global regulatory requirements while dramatically reducing the time to generate submission-ready documentation.
Standard Stability Tables included
Generate all kind of stability tables with automated data transformation from various LIMS sources, ensuring data integrity and regulatory compliance throughout the automated reporting process.
Configurable Report Templates
Configure your template for different purposes – from CofA and initial approval to ongoing stability studies, and ad-hoc reports. Perfect-fit for every stability study phase.
ICH Q1E Shelf-Life Evaluation
Automated shelf-life and shelf-life plots determination following ICH Q1E guidelines, with comprehensive statistical modeling and confidence interval calculations – immediately ready for your approval documentation without any further external systems.
Seamless System Integration
Secure and validated interfaces to LIMS, ELNs, SAP, and other laboratory database systems, ensuring compliant data transfer across your entire digital ecosystem. Specific connectors and automated data mapping, eliminate manual data entry while maintaining full audit trails and 21 CFR Part 11 compliance throughout the reporting process.
Unknown Impurity Control Strategy
Unknown impurities, typos in parameter names, changed method definitions, or country-specific product specs? No problem – StudyReporter Stability catches and fixes all of that right during report creation.
Submission-Ready Output
Equipped with pre-configured report templates for all major regulatory submissions, including INDA, NDA, Annual Reports, and PQR/APR. This ready-to-use framework ensures compliance with global regulatory requirements while dramatically reducing the time to generate submission-ready documentation.
Automated Reports
from any source
in any format
for Stability Studies
Pharmaceutical submission ready reports for stability studies are not conventional documents. They often require extensive compilation activities and qualtiy check procedures to guarantee data integrity. This process is extremely time-consuming, as many values must be manually cross-checked.
With over two decades of expertise in pharmaceutical quality processes, StudyReporter has become the stability reporting partner of choice for QC and CMC departments at leading pharmaceutical companies worldwide. Our automation solutions are specifically designed for the creation of ICH-compliant stability studies and can transform stability data from any LIMS into submission-ready documentation in minutes.
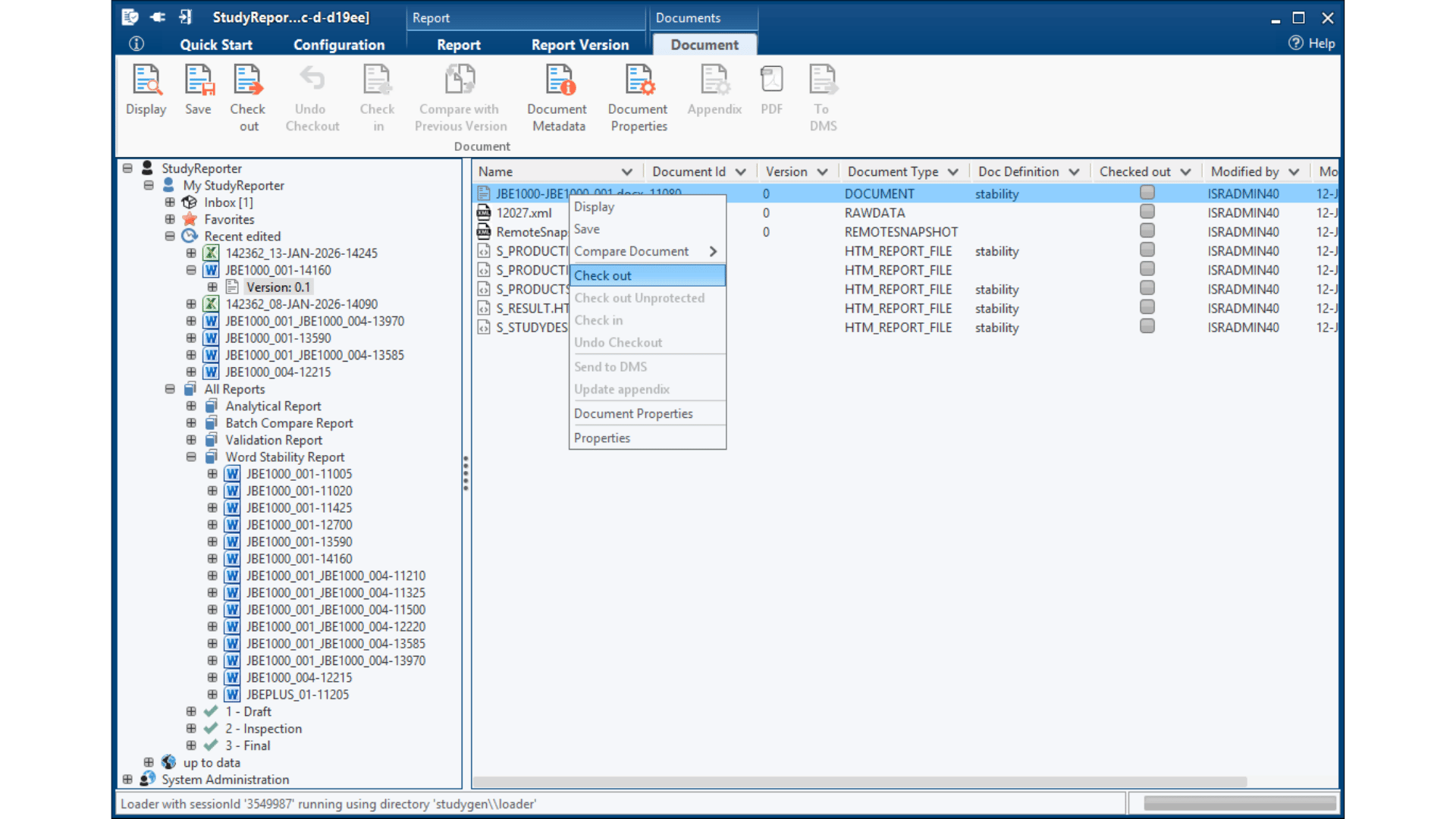
Insights in StudyReporter Stability
Selection of available Stability Tables
Key Benefits
Accelerated Time to Market
Reduce study document and report creation from weeks to hours, getting your products to market faster. This speed advantage directly translates into a competitive edge and earlier revenue generation.
Integrated Compliance
Automated systems eliminate manual data entry errors and ensure ICH-compliant study designs every time. This protects you from costly regulatory rejections and audit findings.
Resource Optimization
Free your skilled scientists from repetitive tasks to focus on high-value lab activities. Cut overall study costs through streamlined workflows and reduced error correction.
Seamless Data Integration
Connect directly with your LIMS, ERP, SAP and QMS systems for automatic data flow. Eliminate duplicate entries and create a single source of truth for all stability data.

Your Journey Starts Here
Ready to transform your stability data reporting?
Experience the power of StudyGen 360’s integrated platform.
Your Solution for Stability Reports
Whether for CofAs, standard stability docuements or ongoing stability reporting, we are trusted partner for report automation.
Your Journey Starts Here
Ready to transform your stability data reporting?
Experience the power of StudyGen 360’s integrated platform.

Your Solution for Stability Reports
Whether for CofAs, standard stability reports or ongoing stability solutions, we are trusted partner for report automation.