Understanding the Stakes:
Cross Run Assay-Control Matters
Bioanalytical laboratories face growing challenges when it comes to maintaining consistent assay quality over the study. The implementation of standardized multi-run assay controls to mitigate risks in bioanalytical analyses is therefore critical to the success of clinical trials.
Understanding the Stakes: Cross Run Assay-Control Matters
Bioanalytical laboratories face growing challenges when it comes to maintaining consistent assay quality over the study. The implementation of standardized multi-run assay controls to mitigate risks in bioanalytical analyses is therefore critical to the success of clinical trials.
rethinking bioanalytical RUN Control
Comprehensive Bioanalytic Quality Assurance before Reporting
Regular QC Checks
Beyond the scope of ICH M10, based on industry best practise: Continuous quality control through automated evaluation of QC samples. Identification of deviations in real time – before they affect the overall study.
Configurable Acceptance Criteria
Flexible definition of acceptance criteria in accordance with FDA/EMA guidelines (±15% for QCs, ±20% for LLOQ). Automatic validation against defined limit values – adaptable to sponsor requirements.
Workflow Optimization
Early detection of problematic runs prevents costly rework. Live tables improve immediate decisions on run acceptance and reduce repeat analyses. Overall this approach reduces the risk of study failure.
Automated Run Documentation
Complete documentation of all data with audit trail. Automatic generation of run acceptance reports and graphical representation of QC trends – all 21 CFR Part 11 compliant.
Regular QC Checks
Beyond the scope of ICH M10, based on industry best practise: Continuous quality control through automated evaluation of QC samples. Identification of deviations in real time – before they affect the overall study.
Configurable Acceptance Criteria
Flexible definition of acceptance criteria in accordance with FDA/EMA guidelines (±15% for QCs, ±20% for LLOQ). Automatic validation against defined limit values – adaptable to sponsor requirements.
Workflow Optimization
Early detection of problematic runs prevents costly rework. Live tables improve immediate decisions on run acceptance and reduce repeat analyses. Overall this approach reduces the risk of study failure.
Automated Run Documentation
Complete documentation of all data with audit trail. Automatic generation of run acceptance reports and graphical representation of QC trends – all 21 CFR Part 11 compliant.
Challenges we Address
A comprehensive approach ensures consistency, reliability, and compliance across multiple assay runs.
Compliance
Data Integrity & Regulatory Excellence
- Maintain consistent data quality standards
- Ensure full GXP-compliance
- Accelerate regulatory approvals
- Meet all acceptance criteria reliably
- Prepare confidently for inspections
Instrument Performance
Optimizing Operations & Capacity
- Detect hidden issues before impacting study results
- Predict optimal maintenance windows
- Maximize ROI through reduced failures
- Enable data-driven decision making
- Immediate cross-run evaluation
Quality Assurance
Strengthening Business Performance
- Safeguard study integrity and quality
- Enhance CRO – sponsor relationships
- Protect financial performance
- Strengthen competitive position
- Build market reputation
Standardized Assay Control Methods
Successful bioanalytical processes need proactive management and control of key performance parameters
Carryover Analysis Check
- Blank sample monitoring post-ULOQ
- Carryover trend identification
- Wash procedure effectiveness
Comprehensive tracking of all laboratory activities with tamper-evident documentation.
Internal Standard Control
- IS response variability tracking
- FDA Guidance 2019 compliance
- Matrix effect evaluation
Quality Beyond ICH M10
- Method performance qualification
- Procedural robustness an ruggedness
- Detection / Quantification ensurance
Automated Live Tables
from any Source
in any Format
for Study Performance Checks
With decades of specialized experience in bioanalytical data management, StudyGen 360 has established itself as the preferred quality control platform for leading bioanalytical laboratories worldwide.
Our platform implements comprehensively solutions specifically for multi-run assay control and statistical quality management across complex bioanalytical studies.
Always monitor the quality of measurement of your study and base your decisions in real-time as soon as new results are available from your samples.
REAL-TIME STUDY REPORTING
Live Views for Proactive Decisions
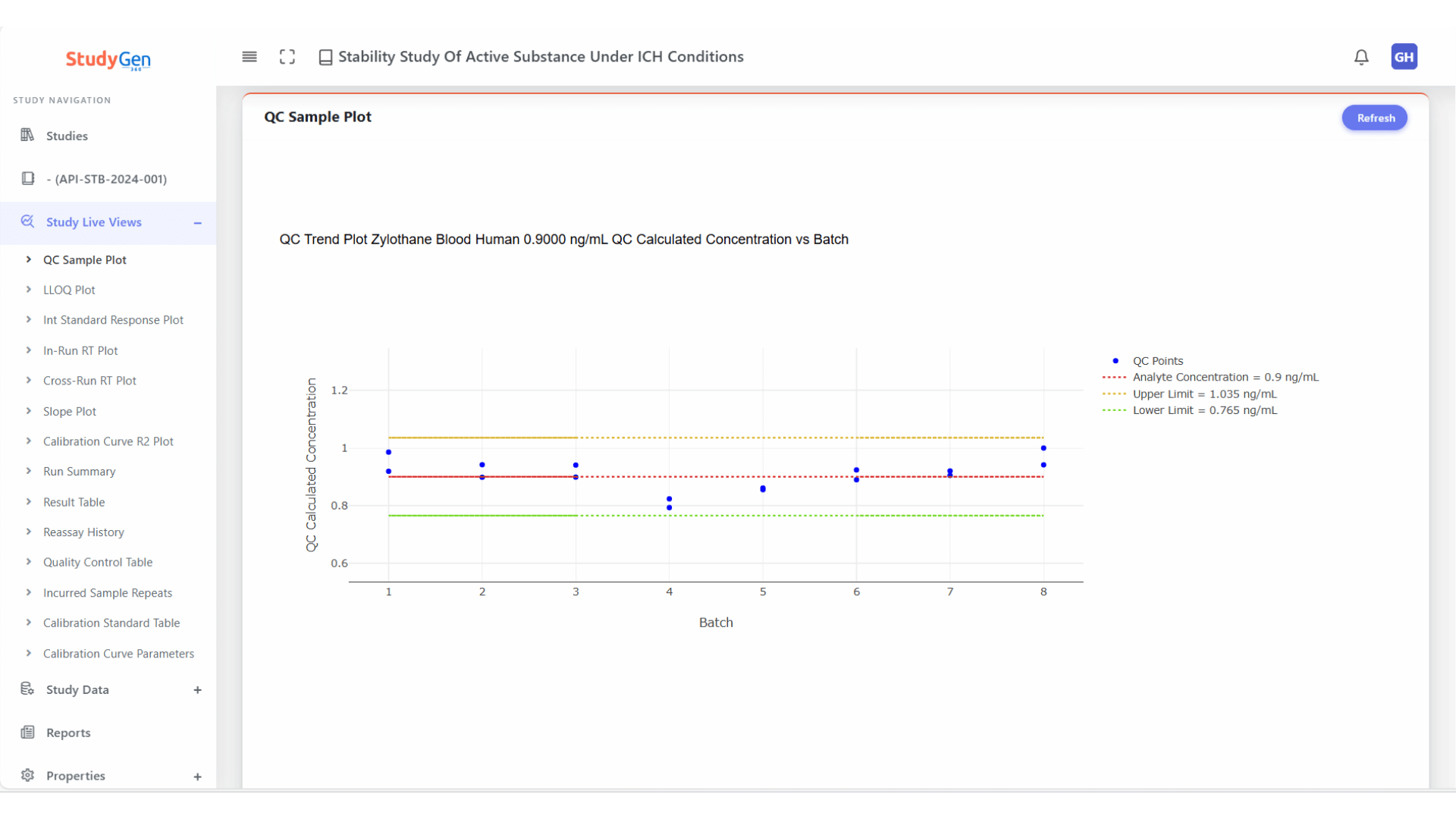
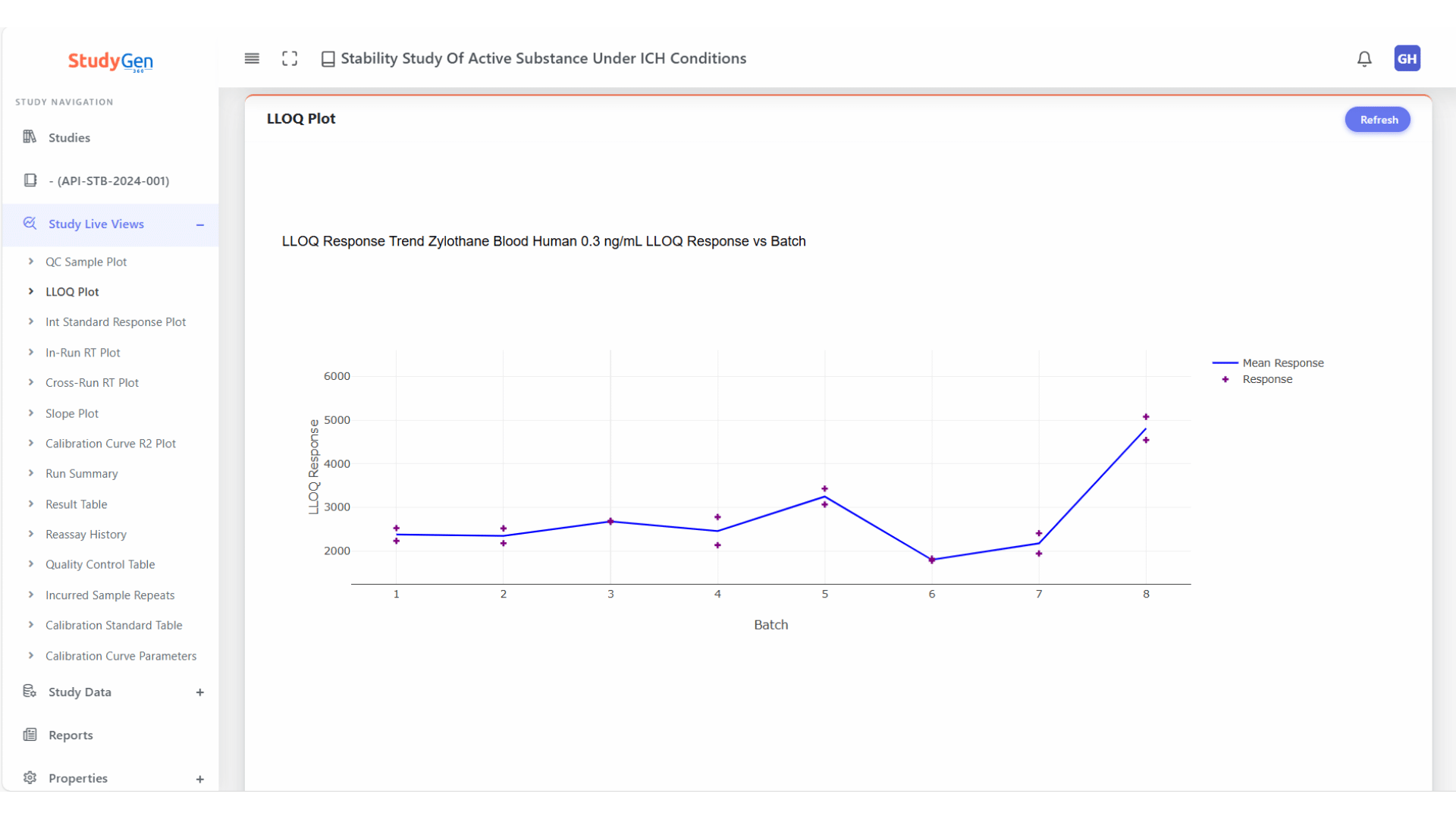
QC Trend Analysis
Continuous monitoring of QC samples across all runs with automatic visualization of control limits and deviations.
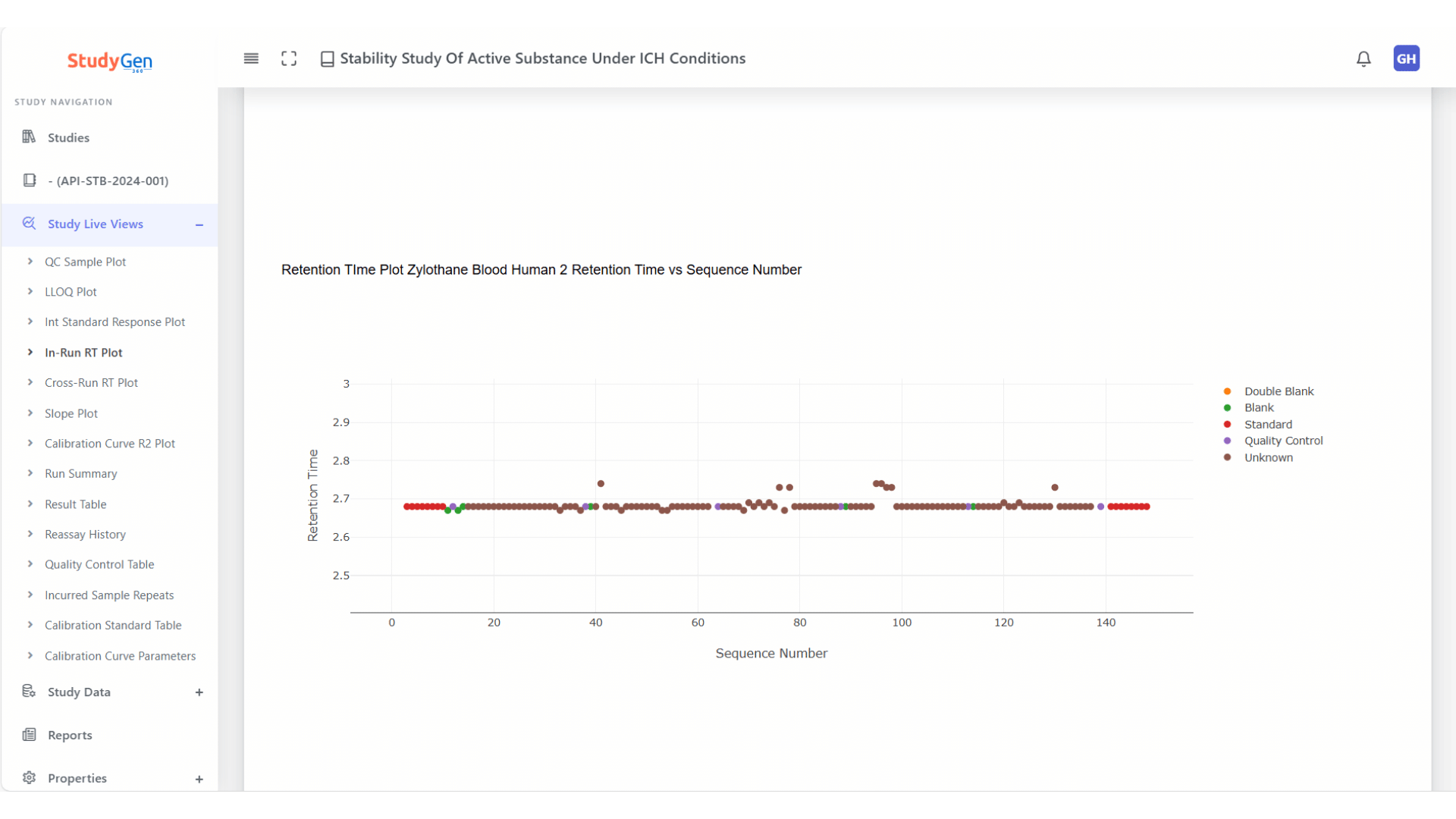
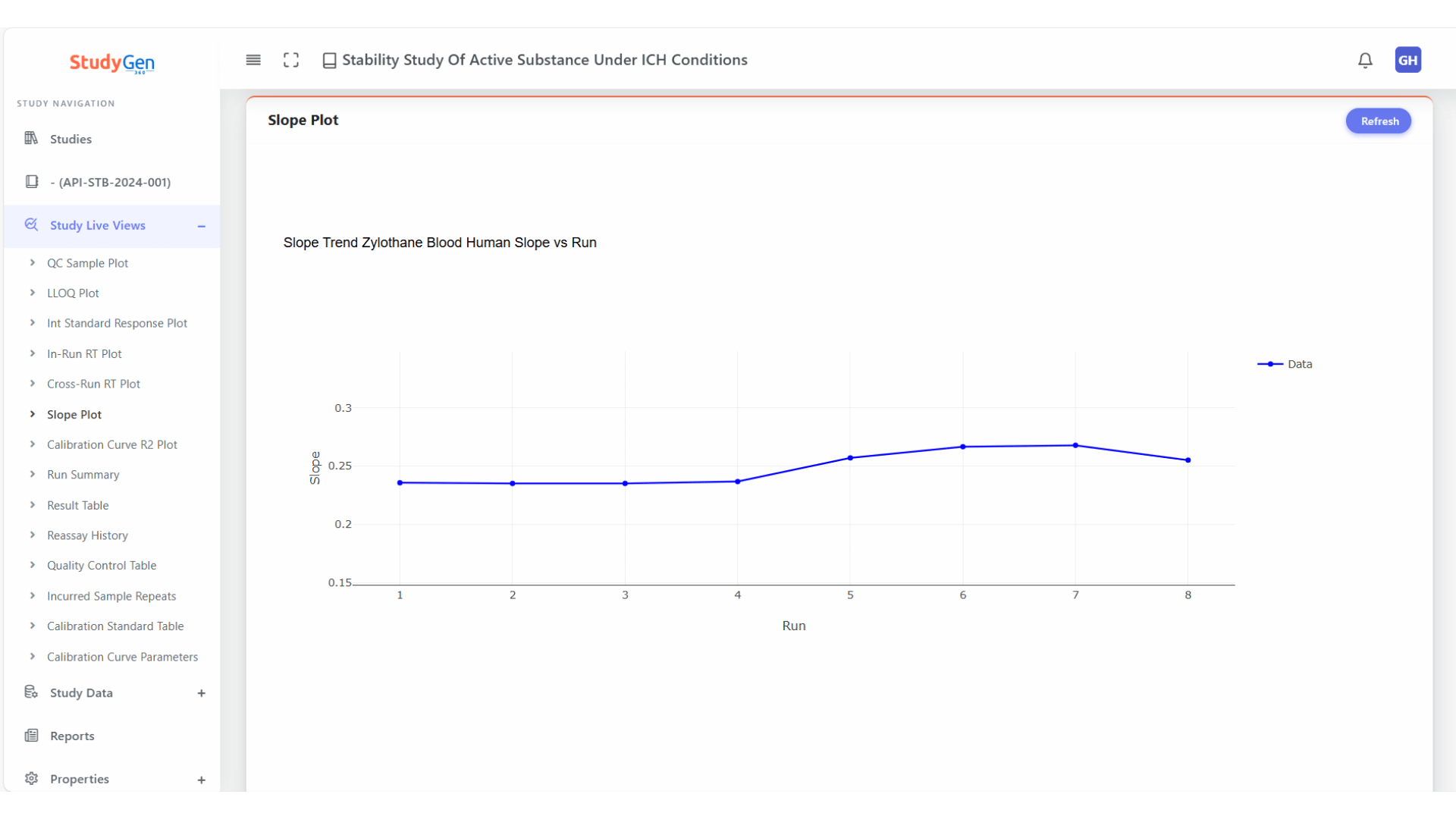
Chromatographic Stability
In-run and cross-run retention time monitoring for early detection of drift, shift, or chromatographic instabilities.
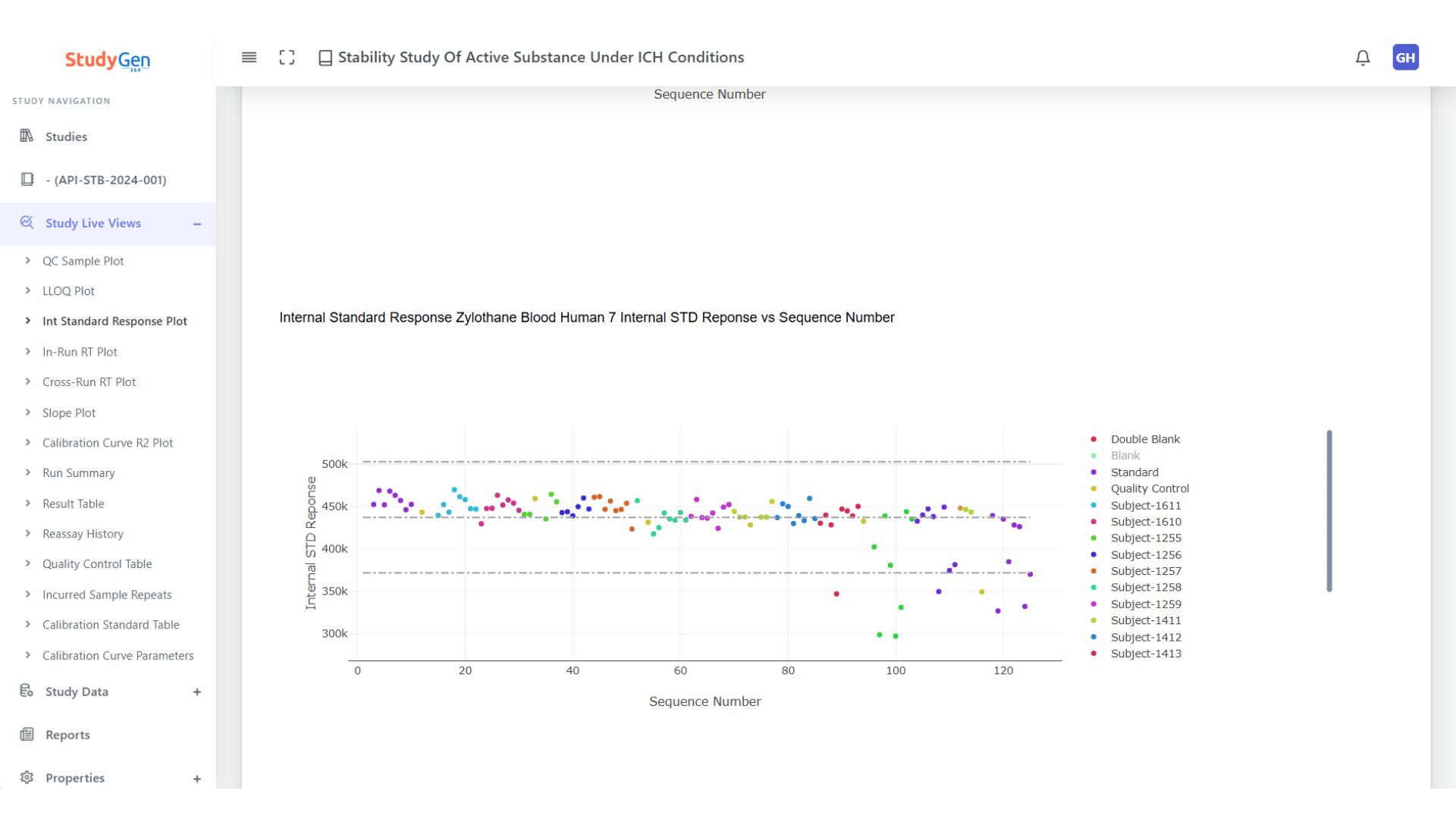
Internal Standards Control
Following the FDA guidance “Evaluation of Internal Standard Responses during Chromatographic Bioanalysis”.

Continued Batch Monitoring
QC trends, retention time, and responses in real-time – automatic visualization of control limits and threshold exceedances.
Instrument Performance
Cross-run analyses for early detection of systematic issues – immediate run assessment for faster decisions during ongoing studies.

Your Journey Starts Here
Ready for Standardized Multi-Run Assay Control?
Experience the power of StudyGen 360’s integrated platform.
Scalable Solution for any Lab Size
Whether you’re a small research lab or a large pharmaceutical company, StudyGen 360 scales with your needs. Learn more here!
Your Journey Starts Here
Ready for Standardized Multi-Run Assay Control?
Experience the power of StudyGen 360’s integrated platform.

In short:
Scalable Solution for any Lab Size
Whether you’re a small research lab or a large pharmaceutical company, StudyGen 360 scales with your needs. Learn more here!