Accelerating Drug Development with Advanced Study Data Management!
From biologics to traditional drugs, StudyGen 360 streamlines clinical studies through intelligent data management and automated reporting while ensuring data integrity and regulatory compliance.
Accelerating Drug Development with Advanced Study Data Management
From biologics to traditional drugs, StudyGen 360 streamlines clinical studies through intelligent data management and automated reporting while ensuring data integrity and regulatory compliance.
rethinking bioanalytical report generation
StudyGen360 – The Game-Changer for LC-MS & LBA Bioanalytical Studies
Standard Reports 100% Automated
Bioanalytical and validation reports are generated fully automatically – compliant with FDA MV-Guidelines and ICH M10. No more manual data entry, no transcription errors, direct integration from your LIMS or instrument data.
Configurable Report Tables
Adapt your report tables to different study designs and sponsor requirements. From traditional small molecules to complex biologics – StudyGen360 supports all assay types with individually configurable layouts.
Workflow Optimization
Instant report generation without time-consuming data cross-checks. Connect your sponsor and provide automated live tables – provide quality-relevant information in real-time at every study timepoint, for maximum transparency and efficiency.
Submission ready Output
We work with your templates – for sponsors and CROs alike. The result: submission-ready reports that meet all regulatory requirements, from first draft to final document.
Complete Audit Trail
Full traceability of all changes with electronic signatures compliant with 21 CFR Part 11. Every calculation and data modification is documented and traceable for audits at any time.
Massive Time Savings
Reduce your report generation time by up to 80%. Automatic data transfer, live updates, and pre-configured templates eliminate manual entries and minimize error sources.
Standard Reports 100% Automated
Bioanalytical and validation reports are generated fully automatically – compliant with FDA MV-Guidelines and ICH M10. No more manual data entry, no transcription errors, direct integration from your LIMS or instrument data.
Configurable Report Tables
Adapt your report tables to different study designs and sponsor requirements. From traditional small molecules to complex biologics – StudyGen360 supports all assay types with individually configurable layouts.
Workflow Optimization
Instant report generation without time-consuming data cross-checks. Connect your sponsor and provide automated live tables – provide quality-relevant information in real-time at every study timepoint, for maximum transparency and efficiency.
Submission ready Output
We work with your templates – for sponsors and CROs alike. The result: submission-ready reports that meet all regulatory requirements, from first draft to final document.
Complete Audit Trail
Full traceability of all changes with electronic signatures compliant with 21 CFR Part 11. Every calculation and data modification is documented and traceable for audits at any time.
Massive Time Savings
Reduce your report generation time by up to 80%. Automatic data transfer, live updates, and pre-configured templates eliminate manual entries and minimize error sources.
Industry Challenges We Solve
Meeting Complex Regulatory Requirements
Easily navigate the complex regulatory landscape that ensures your studies comply with FDA, EMA, and ICH guidelines:
- Standardized data and report formats
- Full Set of ICH M10 standard tables
- Built-in validation rules
- Automated audit tracking
- 21 CFR Part 11 compliant
Multi-Source Data Integration
The lab landscape remains heterogeneous due to varying lab sizes and diverse equipment configurations across facilities.
- Modular solutions
- Universal APIs for instruments and other systems such as LIMS, ELN, SDMS
- Low code configurations
- Tiered pricing options
Accelerate Timelines while keeping Compliance
Keep your team focused on science, not on extensive paperwork and double checks.
- Automated data processing streamlining existing lab workflows
- Full data integrity
- Real-time monitoring through live tables
- Instant GxP-report generation
Automated Reports
from any source
in any format
for small and large Molecules
Pharmaceutical submission ready reports for small and large molecules are not conventional documents. They often require extensive compilation activities and qualtiy check procedures to guarantee data integrity. This process is extremely time-consuming, as many values must be manually cross-checked.
With over two decades of specialized experience in bioanalytical demands, StudyGen 360 has established itself as the preferred study data management partner for leading pharmaceutical companies worldwide. Our platform seamlessly handles the complexities of both large molecule biologics and small molecule drug development, ensuring regulatory compliance while accelerating time-to-market for breakthrough therapies.
Comprehensive Data Management & Regulatory Reporting Made Simple
Insights in StudyReporter based on LC-MS Data
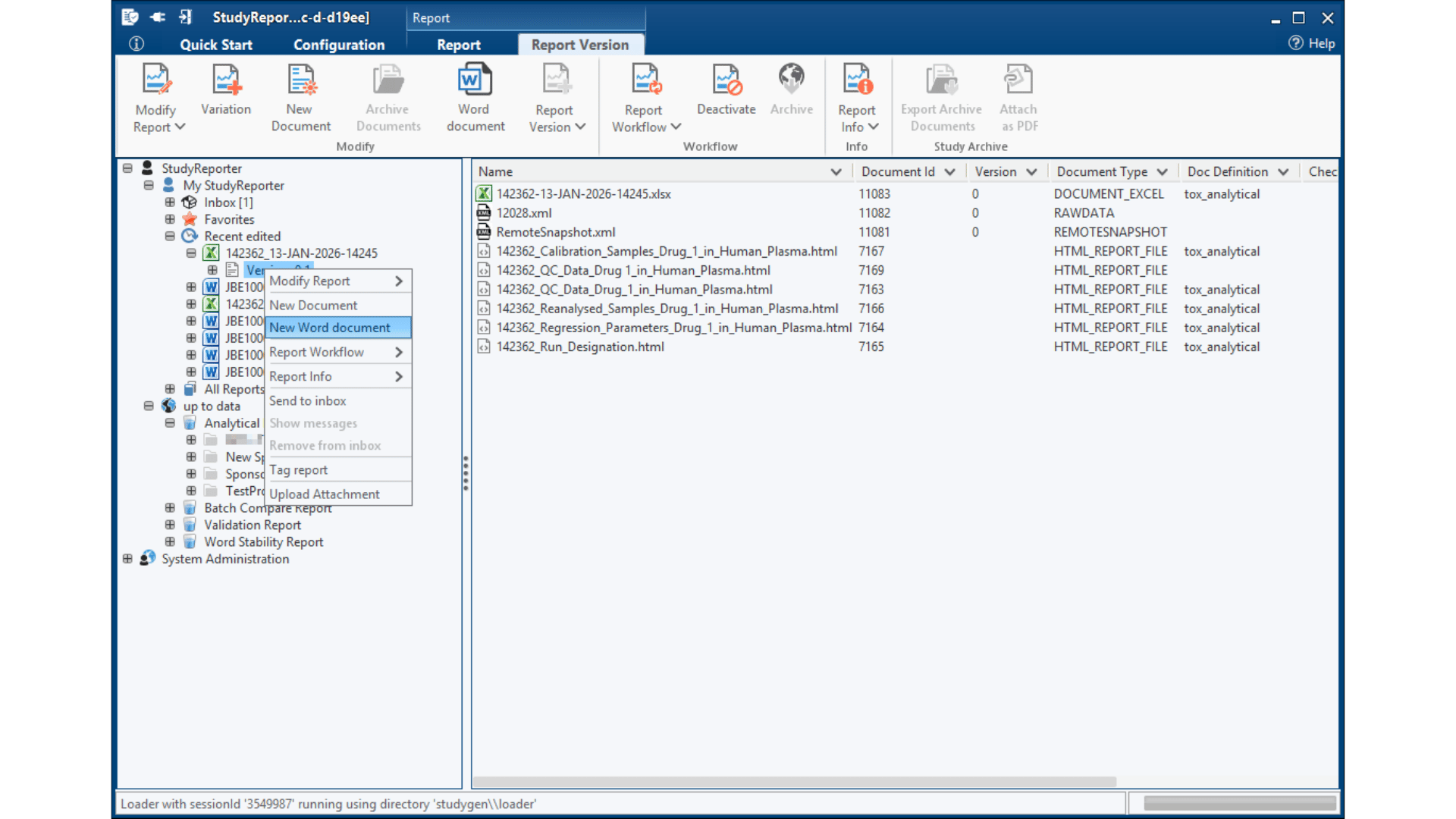
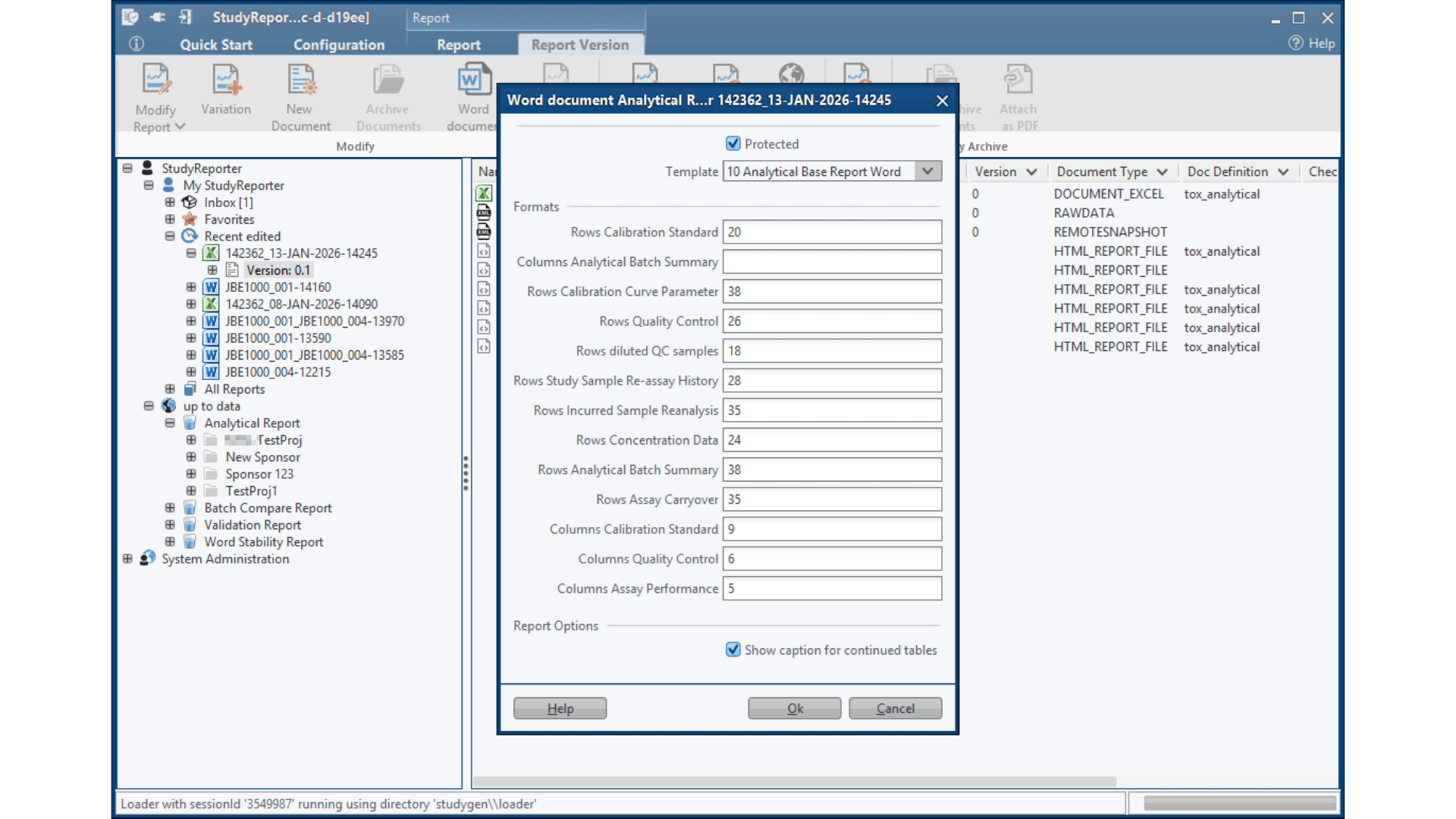
StudyGen 360 Reporter fundamentally impacts bioanalytical study reporting processes by automatically integrating your LC-MS chromatographic data and LBA results into ICH M10-compliant reports in minutes, not weeks.
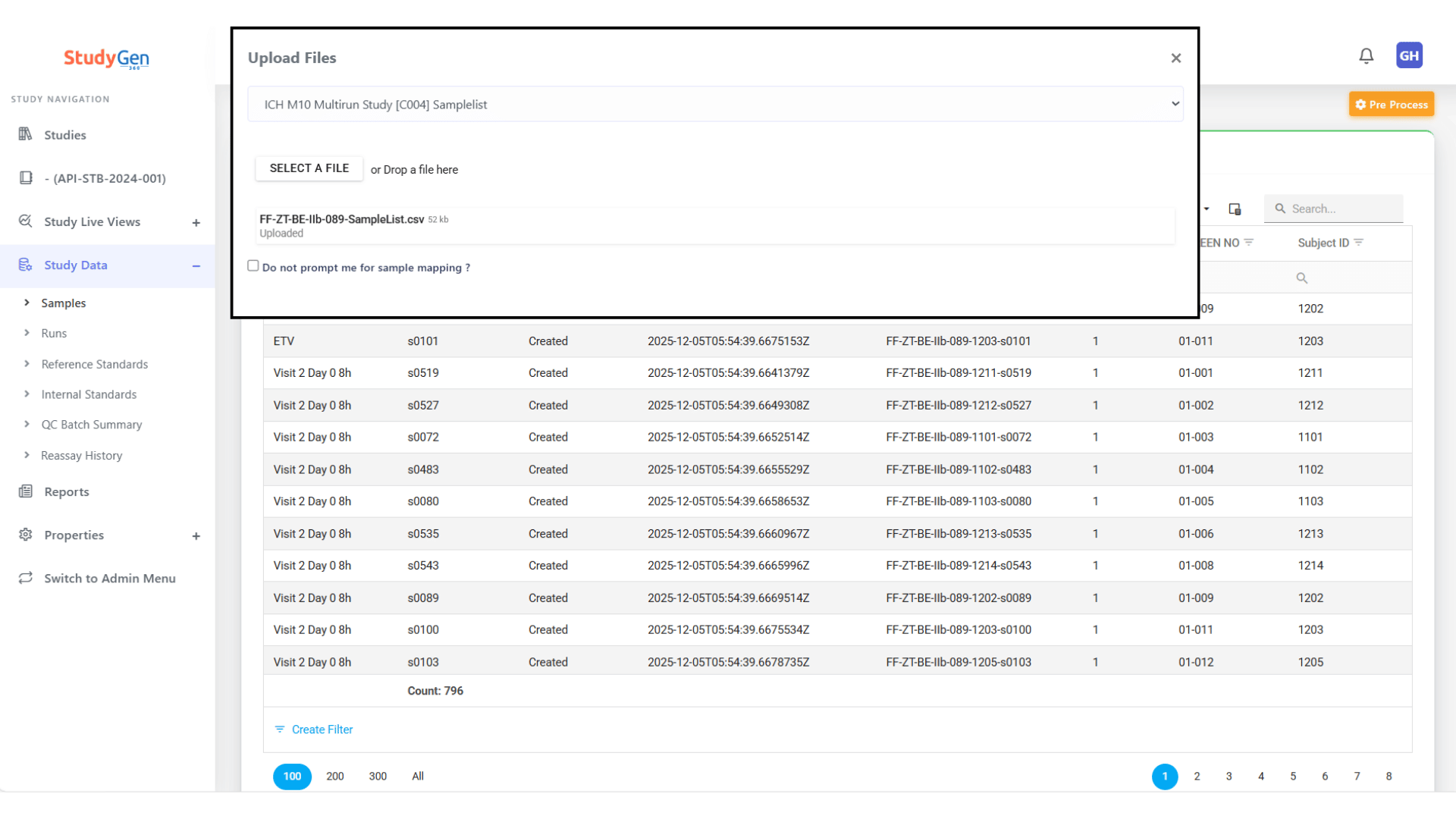
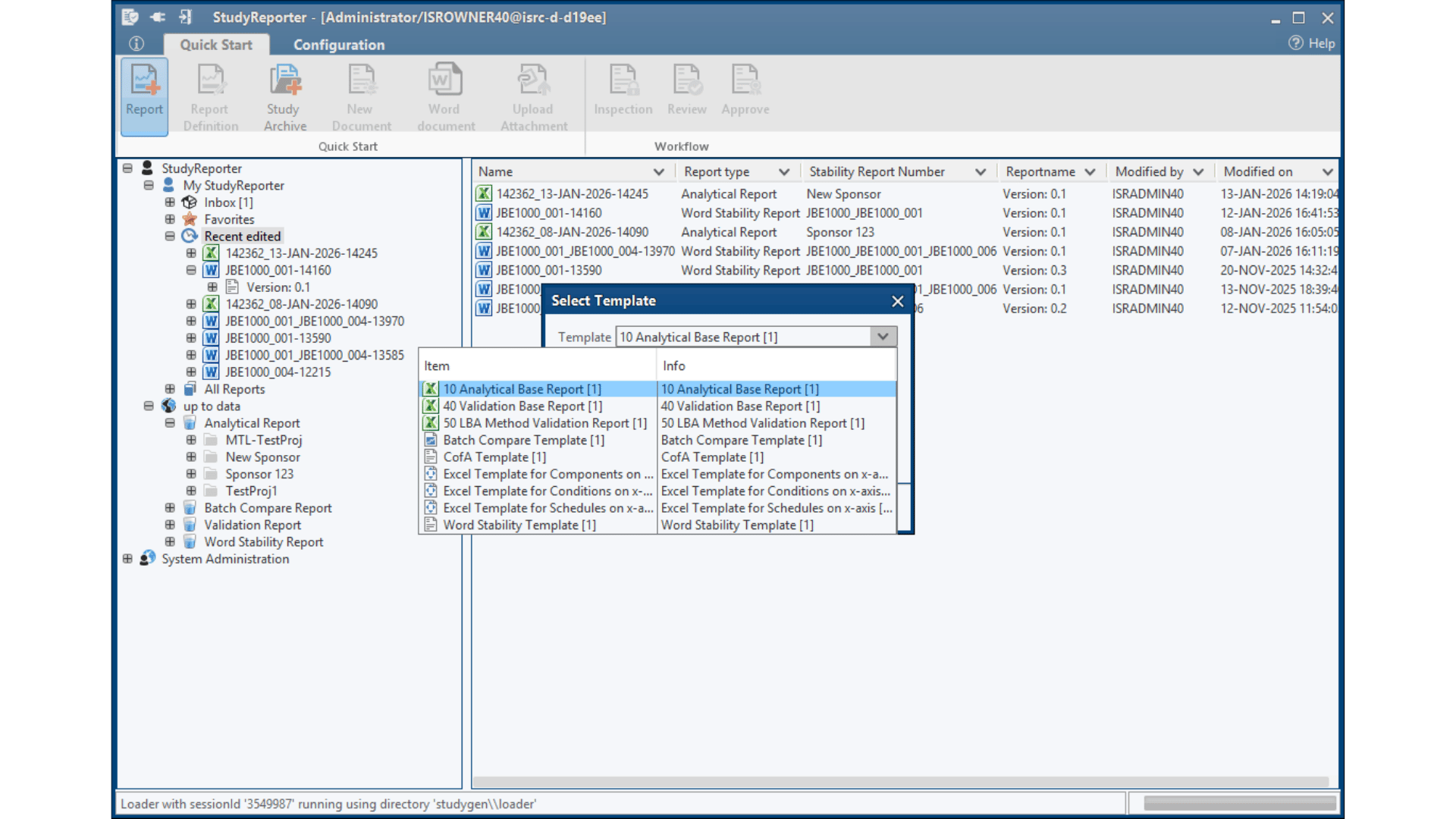
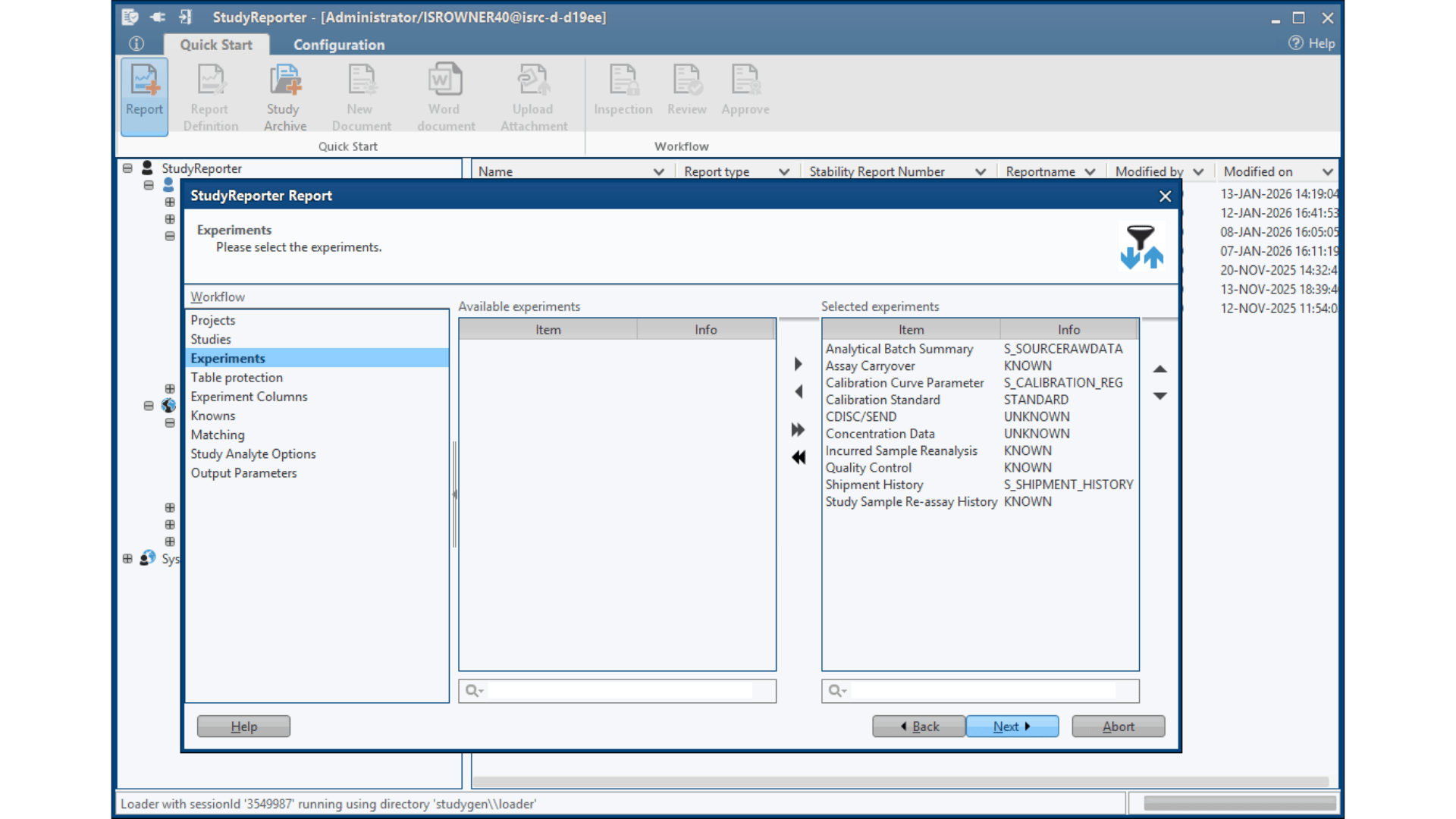
Study Setup & Data Import
– Automatic data integration from LC-MS/LIMS
– Drag & drop interface for various data sources
– Template selection for different study types

QC Sample Monitoring
– Trend-Analyse acreoss all runs
– Automatic outlier detection
– Control charts per ICH M10
– Three-level QC tracking

Calibration Standards Overview
– LLOQ response trends
– Signal-to-noise ratio monitoring
– Calibration curve assessment
– Acceptance criteria visualization
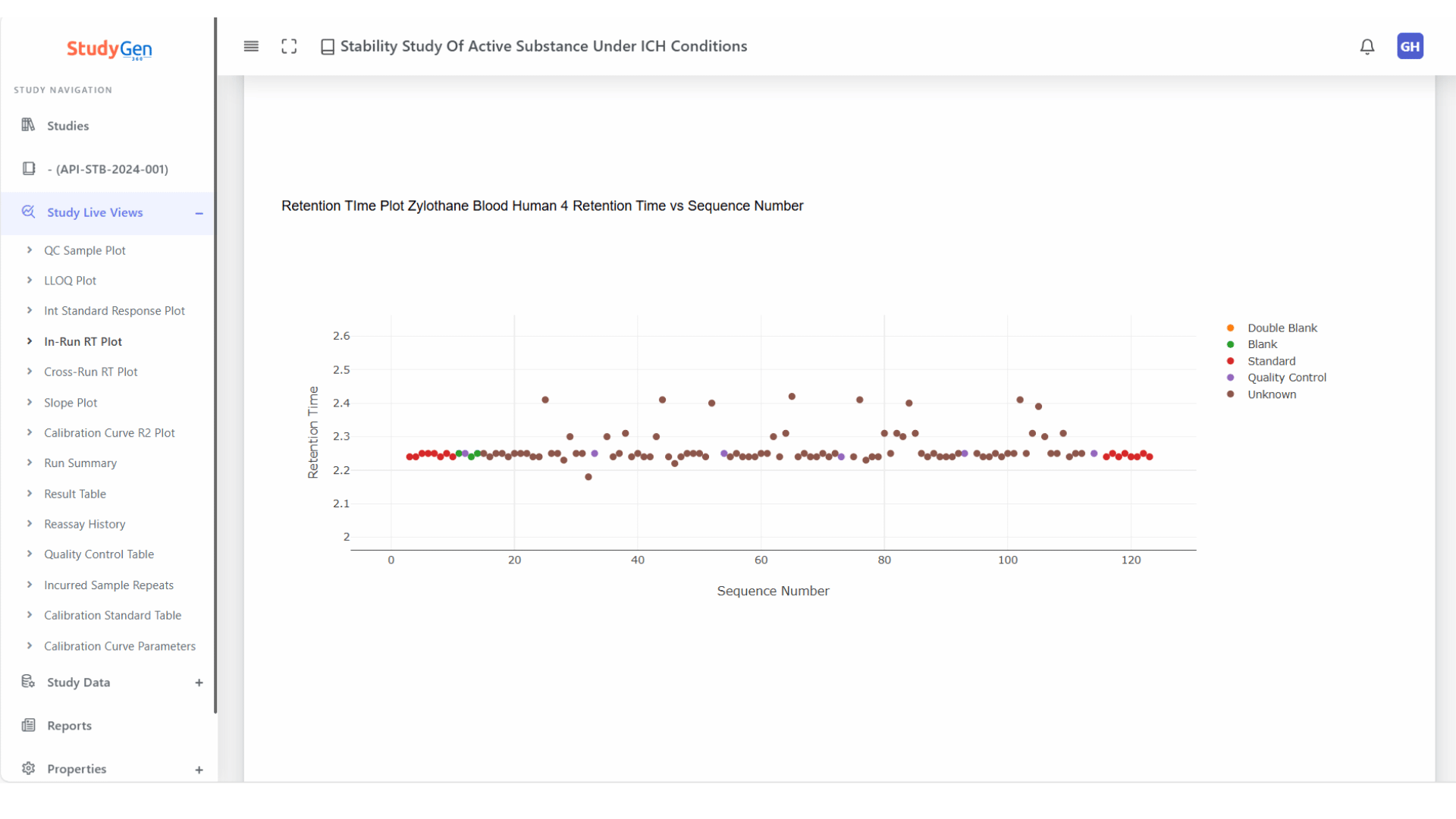
Retention Time Control
– In-run consistency checks
– Cross-run variability analysis
– Automated deviation flagging
Insights in Thermo Scientific™ Watson LIMS™ Standard Reporting*
StudyReporter has established itself as the industry-standard solution for Thermo Scientific™ Watson LIMS™ reporting, trusted by pharmaceutical companies and CROs worldwide for comprehensive study documentation. With years of proven deployment across enterprise environments, it has become the go-to platform for organizations requiring robust, compliant reporting capabilities in their drug development workflows.
*also available for other LIMS solutions – ask us about integration with your specific solution!
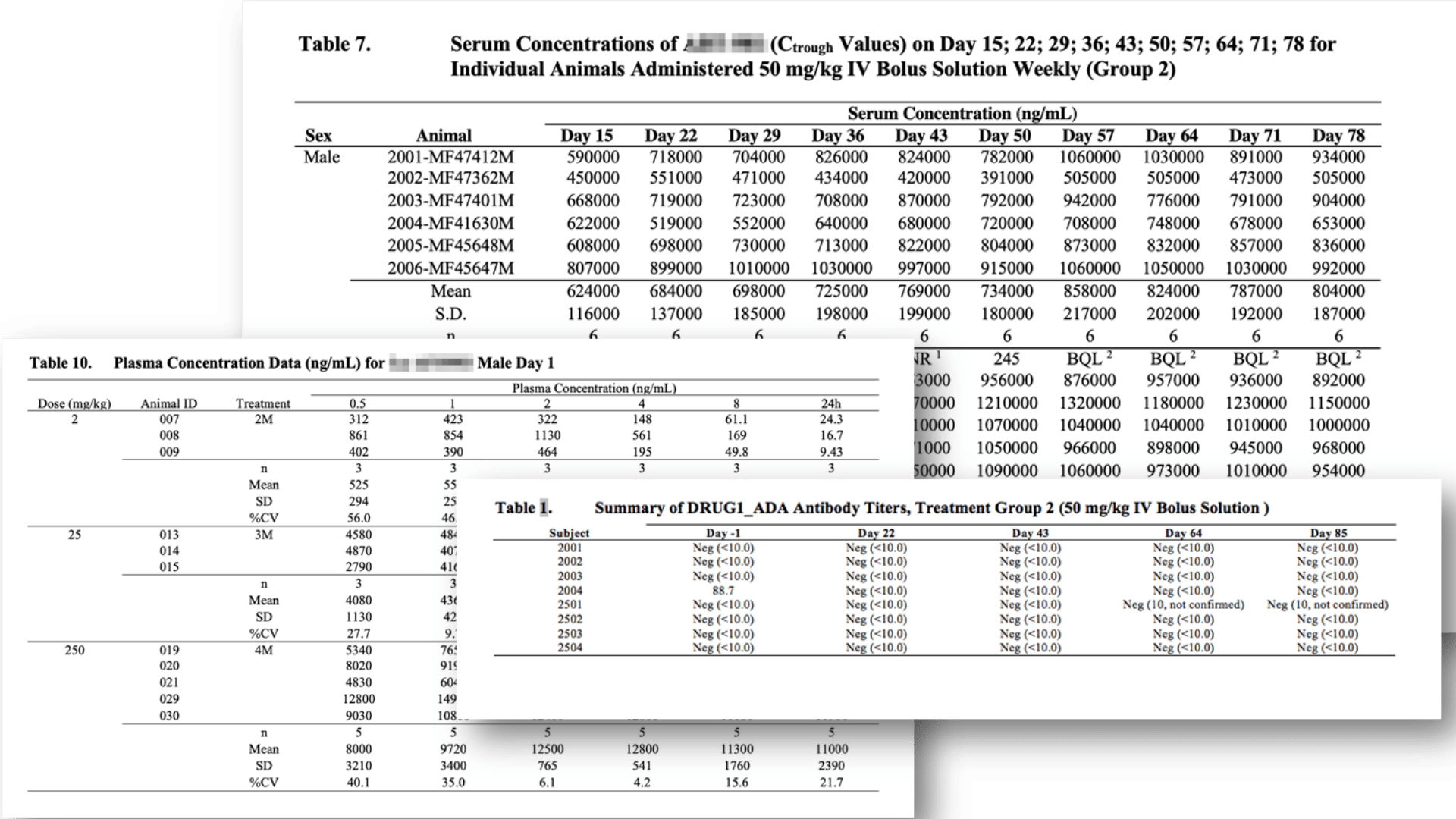
Selection of Bioanalytic ICH M10 Standard Tables
Key Benefits
Accelerated Time to Market
Reduce study document and report creation from weeks to hours, getting your products to market faster. This speed advantage directly translates into a competitive edge and earlier revenue generation.
Integrated Compliance
Automated systems eliminate manual data entry errors and ensure ICH M10 compliant study designs every time. This protects you from costly regulatory rejections and audit findings.
Resource Optimization
Free your skilled scientists from repetitive tasks to focus on high-value lab activities. Cut overall study costs through streamlined workflows and reduced error correction.
Intelligent Issue Resolution
Proactively detect and resolve critical quality issues in real-time – from QC failures and retention time shifts to calibration deviations – using integrated Multi Run QC inspections across all live tables
Seamless Data Integration
Connect directly with your Instrument or LIMS, ELN systems for automatic data flow. Eliminate duplicate entries and create a single source of truth for all lab data.
Rapid GxP-Reporting
Generate GxP bioanalytical reports in minutes. Start reporting immediately, enrich with additional context, and maintain consistent comment sections that remain unchanged during report updates for maximum efficiency.

Your Journey Starts Here
Ready to streamline your large & small molecule studies?Experience the power of StudyGen 360’s integrated platform. Ready to transform your clinical trials data management?
Scalable Solution for any Lab Size
Whether you’re a small research lab or a large pharmaceutical company, StudyGen 360 scales with your needs. Learn more here!
Your Journey Starts Here
Ready to streamline your large & small molecule studies?Experience the power of StudyGen 360’s integrated platform. Ready to transform your clinical trials data management?
Experience the power of StudyGen 360’s integrated platform.

Scalable Solution for any Lab Size
Whether you’re a small research lab or a large pharmaceutical company, StudyGen 360 scales with your needs. Learn more here!